Industry: Healthcare
Published Date: February-2025
Format: PPT*, PDF, EXCEL
Delivery Timelines: Contact Sales
Number of Pages: 250
Report ID: PMRREP33246
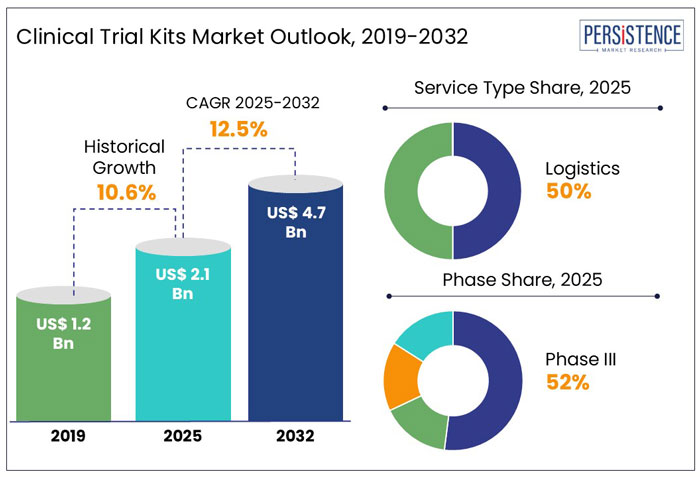
The global clinical trial kits market was valued at around US$ 1.9 Bn in 2024. With a projected CAGR of 12.5% for the next ten years; the market is likely to reach a valuation of US$ 4.7 Bn by the end of 2032. The increasing number of clinical trials performed globally is driving the clinical trial kits market.
|
Report Attributes |
Details |
|
Estimated Market Value (2025E) |
US$ 2.1 Bn |
|
Forecasted Market Value (2032F) |
US$ 4.7 Bn |
|
Global Market Growth Rate (2025-2032) |
12.5% CAGR |
|
United States Growth Rate (2025-2032) |
13.2% CAGR |
Persistence Market Research’s analysis reveals that most of the market revenue is grossed from logistics service-type clinical trial kits. On the other hand, clinical trial kits for phase III will be the most lucrative with an 11.4% of CAGR during 2025 - 2032.
The global market for clinical trial kits expanded with a CAGR of 10.6% during 2019-2024. The global clinical trial kits market is expected to create an absolute dollar opportunity of more than US$ 4.7 Bn during 2025-2032.
In 2024, the global market size of clinical trial kits was valued at US$ 1.9 Bn and is estimated to increase to a valuation of US$ 2.1 Bn in 2025. The increasing technological advancement in clinical trials has increased the use of clinical trial kits. Furthermore, the rise in the use of home-based testing has led to further medical research being pursued remotely. As testing is made possible through remote technology and increased adoption, it will help to alleviate doctors' workloads, as is made clear during frequent testing and patient monitoring. Increasing the use of remote testing and considering virtual technologies by professionals is expected to drive the market for medical research.
Additionally, As of May 2023, the ClinicalTrials.gov database recorded the following number of registered studies across different clinical phases: Early Phase I/Phase I: 58,207 studies (28%), Phase II: 74,432 studies (36%), Phase III: 42,947 studies (21%), and Phase IV: 30,894 studies (15%). The growing number of clinical trials across all phases highlights the increasing demand for essential supplies, including clinical trial kits. This rising trend is expected to drive market growth in the coming years.
The global clinical trial kits market is predicted to grow ahead at a CAGR of 12.5% and sales worth US$ 4.7 Bn during 2025 - 2032. The US will continue to be the largest user of clinical trial kits throughout the analysis period accounting for over US$ 1.2 Bn absolute dollar opportunity in the coming 10-year epoch.
The advantage of DtP clinical trials as they are cost-effective and easy to conduct comparing traditional clinical trials has been motivating the pharmaceuticals and drug manufacturing companies to choose DtP clinical trials. In DtP clinical trials samples are collected from the patient’s home and then transferred to the testing facilities and the drugs are directly delivered to the patient’s home. Quality trial kits are to be used because the damaged trial drugs can have big consequences. The demand for DtP clinical trials is increasing which is propelling the growth of the clinical trial kits market.
The transportation of the samples and medicines with optimum temperature conditions is a key factor in the clinical trials. The proper delivery of the samples to the testing setup and the delivery of the drugs to the right patient at right time is important.
Certain samples and medicines should be needed to preserve at a certain temperature and temperature-controlled shipping containers are more expensive to use than standard shippers are. Another challenge is that the chances of error are high when the individuals do the sample collection. These factors are expected to restrain the growth of the clinical trial kits market.
How is the demand for Clinical Trial Kits shaping up in North America?
The rising prevalence of cancer across North America has significantly driven the demand for Phase III clinical trials, as pharmaceutical companies and research institutions seek to develop more effective and targeted therapies. Furthermore, the market for clinical trial kits is dominated by North America, which held around 40.0% of the market in 2024. The presence of top pharmaceutical companies, substantial investments in R&D, and sophisticated healthcare infrastructure all contribute to this strong position. The area gains from advantageous government regulations that expedite regulatory clearances and promote clinical trial innovation. Additionally, trial accessibility and efficiency are improved by the quick deployment of cutting-edge technologies. North America's dominance in the clinical trial kits market is cemented by the growing need for home-based testing solutions and decentralized studies.
US Clinical Trial Kits Market Analysis
According to Clinical Trials.gov, about 32% of the total registered clinical studies are performed in the US. The rapid adoption of decentralized clinical trials is driving the clinical trial kits market in the US. The clinical trial kits market in the US is expected to reach a valuation of US$ 1.2 Bn by 2032.
The rising focus on patient-centered trials, along with the growing burden of chronic conditions such as cancer, cardiovascular diseases, and infectious illnesses, is further fueling demand for clinical trial kits in the U.S. Additionally, regulatory bodies like the FDA are promoting remote and hybrid trial approaches to improve accessibility for a broader patient base. The adoption of advanced digital health solutions, including wearable devices and telemedicine, is also enhancing trial efficiency and data collection, contributing to the expansion of the clinical trial kits market in the region.
China Clinical Trial Kits Market Analysis
The Clinical Trial kits market in China is forecasted to reach a valuation of US$ 385.3 Mn by the end of 2032. China has developed a clinical trial infrastructure with skilled experts and a well-developed network of experienced therapeutic experts. In addition, the large number of the patient pool who are eligible for the clinical trial is also driving the market in China.
Market expansion is also being accelerated by the Chinese government's pro-business legislative changes and higher spending on pharmaceutical research.
Why Logistics service type is driving the demand for clinical trial kits?
The increasing number of clinical trials performed and the need to transport the samples and medicines without any damage are boosting the growth of this segment. The main function of the logistics in the clinical trial process is to transfer the samples to labs very quickly as possible. The logistics segment accounted for a CAGR of 11.3% during 2019-2024.
How is Phase III of clinical trials driving the clinical trial kits market?
Phase III study of clinical trials includes a large patient population and is conducted in many places at the same time is the key factor driving the market. Phase III studies generally take longer times compared to Phase I and Phase II studies. Phase III studies can detect the side effects of the specific drugs, which passed the phase II, study of the clinical trial. The market through Phase III clinical trials accounted a CAGR of 9.1% during 2019-2024.
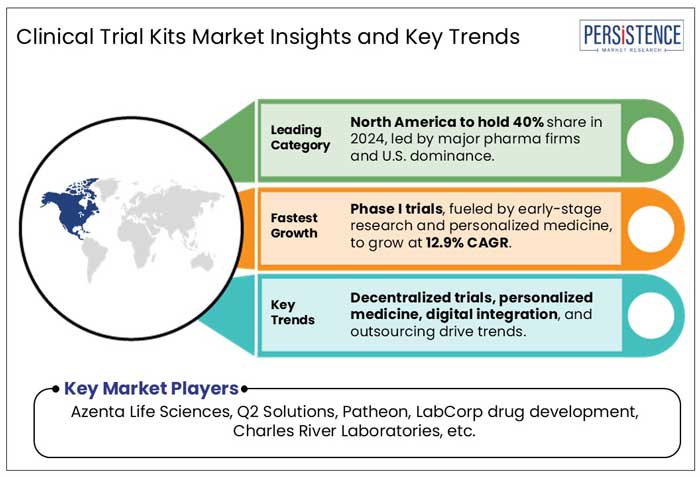
At present, clinical trial kit manufacturers are largely actively expanding their manufacturing facilities to boost production. The key companies operating in the clinical trial kits market include Azenta Life Sciences, Q2 Solutions, Patheon (Thermo fisher scientific), LabCorp drug development, Charles River Laboratories, LabConnect, Almac group, Precision medicine group, Cerba research, Alpha Laboratories Ltd, Marken SAS, and Clinigen.
Some of the recent developments by key providers of clinical trial kits are as follows:
Similarly, the team at Persistence Market Research has tracked recent developments related to companies manufacturing clinical trial kits, which is available in the full report.
|
Attribute |
Details |
|
Forecast Period |
2025-2032 |
|
Historical Data Available for |
2019-2024 |
|
Market Analysis |
|
|
Key Regions Covered |
|
|
Key Market Segments Covered |
|
|
Key Companies Profiled |
|
|
Report Coverage |
|
|
Customization & Pricing |
|
By Service Type:
By Phase:
By Region:
To know more about delivery timeline for this report Contact Sales

The global clinical trial kits market was valued at US$ 2.1 Bn in 2025.
The global clinical trial kits market is set to witness a high growth rate of 12.5% over the forecasted period and be valued at US$ 4.7 Bn by 2032.
Azenta Life Sciences, Q2 Solutions, Patheon, LabCorp Drug Development, and Charles River Laboratories are the key suppliers of Clinical Trial Kits that are shaping the market.
The US, UK, China, Japan, and South Korea are expected to drive the most sales growth of clinical trial kits.
The market in the US has forecasted to account for over 35% of the global market share in 2032.