Industry: Healthcare
Published Date: March-2025
Format: PPT*, PDF, EXCEL
Delivery Timelines: Contact Sales
Number of Pages: 250
Report ID: PMRREP35178
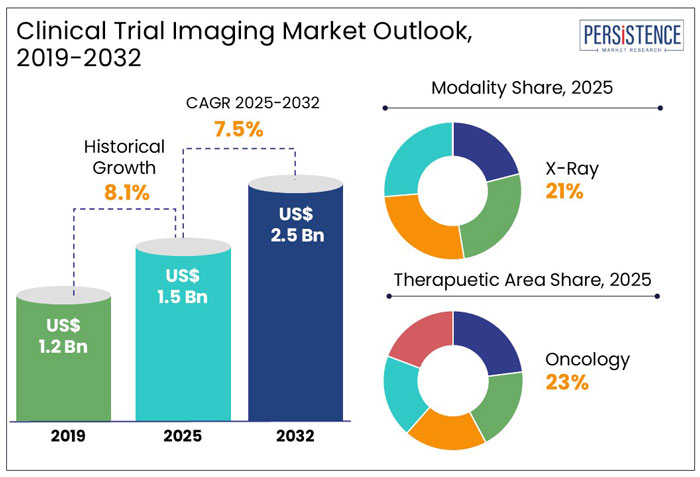
The global clinical trial imaging market size is anticipated to rise from US$ 1.5 Bn in 2025 to US$ 2.5 Bn by 2032. It is projected to witness a CAGR of 7.5% from 2025 to 2032.
The market for clinical trial imaging benefits drug development immensely by providing precise imaging data for assessing treatment efficacy and safety.
The increasing number of trials taking place is boosting the market prospects to witness substantial growth over the forecast period.
The market includes a variety of imaging modalities such as CT, MRI, X-ray, ultrasound, and PET/SPECT, which are commonly utilized in oncology, neurology, cardiology, and other therapeutic fields. The increasing number of clinical trials, advancements in imaging technologies, and expanding adoption of this technology are pushing the market forward.
Pharmaceutical and biotechnology companies, along with contract research organizations (CROs), are the primary users of imaging solutions, leveraging them for biomarker identification, disease monitoring, and regulatory approvals. The rise of decentralized clinical trials, cloud-based imaging systems, and regulatory standards defines the industry's future, creating considerable prospects for innovation and market growth.
Key Highlights of the Market
|
Market Attributes |
Key Insights |
|
Clinical Trial Imaging Market Size (2025E) |
US$ 1.5 Bn |
|
Projected Market Value (2032F) |
US$ 2.5 Bn |
|
Global Market Growth Rate (CAGR 2025 to 2032) |
7.5% |
|
Historical Market Growth Rate (CAGR 2019 to 2024) |
7.3% |
Advancements in Imaging Technology Spurred Market Growth Pre-2024
During the historical period from 2019 to 2024, the market for clinical trial imaging has grown significantly over the last decade. The growth of the market was primarily attributed to the advances in imaging technology, increased clinical trial activity, and regulatory emphasis on imaging biomarkers.
AI-driven automation, cloud-based imaging, and decentralized trial solutions will shape the market's future. AI-powered imaging biomarkers will be critical for early illness detection and therapy response evaluation, especially in oncology and neurology studies.
Cloud-based imaging tools will drive decentralized and virtual trials, increasing accessibility while lowering costs. The coming decade will see a data-driven, AI-powered revamp of clinical trial imaging.
Adoption of Sophisticated Imaging Technologies Bolsters Market Growth
Over the forecast period, the market is poised for continued growth with the adoption of sophisticated imaging technologies. Technologies like AI-enabled body scans are expected to rise, facilitating early detection of health conditions and improving patient outcomes. ?
Regions like Asia Pacific are anticipated to witness accelerated growth due to increasing clinical trial activities, expanding healthcare infrastructure, and a growing patient population. Governments and regulatory bodies are likely to implement favorable policies to encourage innovation and efficiency in clinical trials, further propelling market expansion.
Rise of Virtual and Hybrid Clinical Trials Fuels Demand for Remote Imaging Solutions
The growing adoption of virtual and hybrid clinical trials is significantly increasing the demand for remote imaging solutions in the healthcare and pharmaceutical industries. The shift is driven by the need for greater efficiency, accessibility, and patient convenience in clinical research.
Remote imaging technology enables the seamless collection, review, and analysis of medical images without requiring patients to be physically present at trial sites. Through secure cloud-based platforms, clinical researchers can access, monitor, and analyse imaging data in real time, ensuring faster decision-making, improved data accuracy, and enhanced patient compliance.
The innovation not only reduces logistical challenges but also broadens patient recruitment opportunities by allowing participation from diverse geographic locations. As the clinical trial industry continues to embrace digital transformation, the demand for AI-driven imaging solutions will continue to grow, reshaping the future of clinical research and drug development worldwide.
High Costs of Imaging Technology and Services Impedes Market Expansion
Advanced imaging techniques require expensive equipment, skilled personnel, and significant operational costs. The adoption of modern imaging techniques in clinical trials is restricted by high equipment costs, the requirement for trained professionals, and large operational expenses.
MRI, PET, and 4D imaging require cutting-edge scanners, dedicated imaging centres, and highly trained radiologists, which raises overall trial expenses. Maintenance, software upgrades, and compliance with regulatory standards all contribute to cost constraints.
The high-cost issues are especially acute in underdeveloped or emerging countries, where access to cutting-edge imaging infrastructure is scarce. High costs and resource constraints may cause trial delays and lower acceptance of AI-driven imaging solutions, limiting overall market growth.
Remote and Decentralized Imaging in Hybrid Trials Propels Market Demand
The shift towards hybrid and decentralized clinical trials presents a significant opportunity for remote imaging solutions. Traditional clinical trials require patients to visit trial sites for imaging procedures, which can be a barrier, especially for those in rural or underserved areas.
Cloud-based imaging platforms, mobile imaging units, and telemedicine integration are now enabling patients to participate in trials from remote locations. The patient-centric approach improves recruitment, retention, and data diversity, making trials more inclusive and representative.
Secure cloud storage and AI-driven image processing ensure that imaging data can be accessed and analysed in real-time, reducing delays and enhancing trial efficiency. Further, the growing adoption of remote imaging is set to revolutionize the way clinical trials are conducted, making research more scalable, efficient, and globally connected.
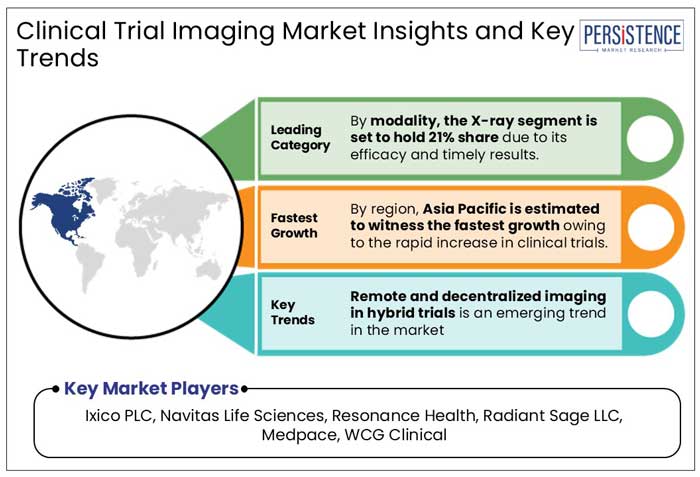
X-Ray Leads with 21% Share Owing to its Efficacy and Timely Results
X-ray imaging remains one of the most widely utilized diagnostic tools in modern healthcare, playing a crucial role in disease detection, treatment monitoring, and clinical trial imaging.
As a standard imaging procedure, X-rays are extensively used to capture internal body structures, particularly bones, allowing healthcare professionals to track disease progression, assess treatment efficacy, and measure critical parameters such as tumour size over time.
In clinical trials, X-ray imaging provides researchers with valuable insights into how a study drug or intervention impacts patients, ensuring data accuracy and improved medical outcomes. The growing reliance on X-rays reflects the increasing demand for advanced imaging technologies as global healthcare access continues to expand.
The rising adoption of cutting-edge X-ray systems is further boosting the clinical trial imaging market. As imaging technology advances, the role of X-rays in clinical trials and precision medicine will continue to evolve, driving innovation in medical imaging solutions worldwide.
Oncology Leads the Therapeutic Area Segmentation with 23% Share Due to growing Number of Cancer Patients Globally
Imaging is a crucial component of cancer research, playing a key role in early detection, disease staging, treatment monitoring, and biomarker evaluation. As cancer rates continue to rise globally, the demand for advanced imaging solutions in oncology clinical trials is growing rapidly.
The rising incidence of cancer has led to a surge in oncology-focused clinical trials, driving the need for high-quality clinical trial imaging to assess the effectiveness of new therapies. As a result, pharmaceutical and biotechnology companies are making significant investments in oncology clinical trials, making this segment the largest revenue contributor in the clinical trial imaging market.
North America holds the largest share of the global market for clinical trial imaging, which is primarily driven by robust R&D investments, advanced healthcare infrastructure, and supportive regulatory policies.
The region continues to lead in AI-powered imaging, machine learning analytics, and cloud-based imaging solutions, transforming the landscape of clinical research and drug development.
North America remains at the forefront of medical imaging advancements, particularly in addressing cardiovascular, neurological, and oncology-related conditions.
The US is a global leader in clinical trials, with major pharmaceutical companies such as Pfizer, Merck, and Johnson & Johnson investing heavily in innovative drug research and precision medicine.
The US has witnessed significant growth in healthcare expenditures, reflecting increased investment in medical research and advanced imaging technologies.
Europe holds a significant market share in the global clinical trial imaging industry, supported by a well-established healthcare infrastructure, stringent regulatory frameworks, and substantial investments in pharmaceutical research.
The region continues to advance in medical imaging technologies, ensuring high-quality standards in clinical trial imaging. Europe remains a hub for clinical research, with a growing number of trials incorporating advanced imaging technologies for precise analysis.
The European Medicines Agency (EMA) has set comprehensive guidelines for imaging use in clinical studies, ensuring high-quality standards and consistency. The EU Clinical Trials Regulation (CTR) has simplified regulatory processes, enabling more efficient cross-border clinical trials and further driving market growth.
As pharmaceutical companies continue to invest in imaging technologies and regulatory bodies streamline trial processes, Europe’s clinical trial imaging sector is set for sustained growth and innovation.
Asia Pacific is emerging as the fastest-growing market for clinical trial imaging, driven by advancements in healthcare infrastructure and a significant increase in clinical trial activity.
Countries such as China and India are making substantial investments in biopharmaceutical research and development, fueling the demand for advanced imaging solutions in clinical trials.
The region has become a global leader in clinical trial volume, benefiting from lower operational costs, faster patient recruitment, and access to diverse patient populations.
As Asia Pacific strengthens its position in global clinical research, the demand for clinical trial imaging solutions will continue to rise, making the region a key driver of innovation and market expansion.
The market for clinical trial imaging is highly competitive, with key players focusing on technological advancements, strategic partnerships, and geographic expansion to strengthen their market presence.
Leading companies in the industry are investing in AI-powered imaging solutions, cloud-based platforms, and advanced analytics to enhance clinical trial efficiency. The market is witnessing a rise in mergers and acquisitions as companies aim to expand service portfolios and improve imaging capabilities.
The growing adoption of remote imaging and real-time data analysis is reshaping the competitive landscape. With the increasing demand for precision medicine and AI-driven diagnostics, companies that prioritize innovation and regulatory compliance are set to gain a competitive edge.
|
Report Attributes |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis Units |
Value: US$ Bn/Mn, Volume: As applicable |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Product & Services
By Modality
By Therapeutic Area
By End User
By Region
To know more about delivery timeline for this report Contact Sales

The market size is set to reach US$ 2.5 Bn by 2032.
The rise of virtual and hybrid clinical trials is one of the key drivers for the market of clinical trial imaging.
North America dominates the market with 28% of the global share.
X-Ray segment is expected to grow rapidly at 7.8% CAGR from 2025-2032.
Ixico PLC, Navitas Life Sciences, Resonance Health, Radiant Sage LLC, and Medpace are few of the leading players in the market.