Industry: Healthcare
Published Date: March-2025
Format: PPT*, PDF, EXCEL
Delivery Timelines: Contact Sales
Number of Pages: 214
Report ID: PMRREP29491
The global autogenous vaccine market is gaining momentum, driven by the increasing demand for customized veterinary vaccines. The market, valued at US$ 152.1 million in 2025, is projected to grow at a CAGR of 5.4%, reaching US$ 219.7 million by 2032. Autogenous vaccines, tailored to specific pathogens from individual herds or flocks, play a crucial role in targeted disease prevention, particularly when commercial vaccines fall short.
Key market trends include advancements in diagnostic and vaccine production technologies, a shift toward precision livestock farming, and growing awareness of antimicrobial resistance. Strategic partnerships are also bolstering market growth.
Evolving livestock management practices, the need for enhanced biosecurity, and rising investments in animal health further support the market. As livestock producers increasingly seek tailored solutions to address specific disease challenges, the autogenous vaccine market is poised for sustained growth in the coming years.
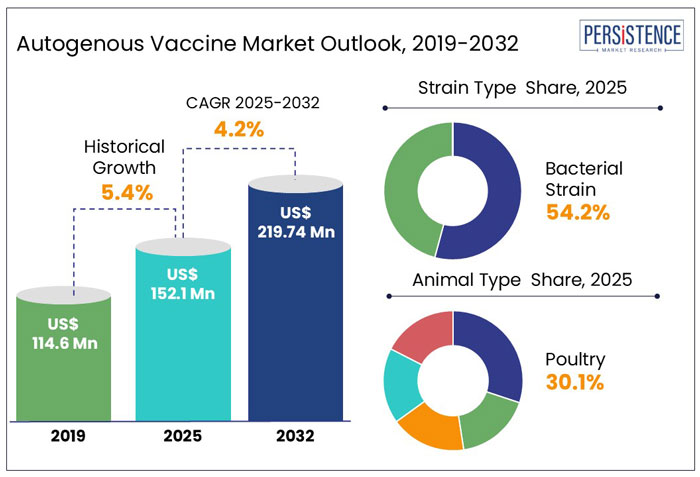
Key Highlights of the Autogenous Vaccine Industry
|
Autogenous Vaccines Market Size (2025) |
US$ 152.1 Mn |
|
Projected Market Value (2032) |
US$ 219.7 Mn |
|
Global Market Growth Rate (2025-2032) |
5.4% CAGR |
| Historical Market Growth (CAGR 2019 to 2024) |
4.2% |
The autogenous vaccine market has evolved significantly, with growing recognition of its role in managing region-specific and herd-specific diseases in livestock. Historically, the market expanded at a CAGR of 4.2%, supported by increasing awareness among veterinarians and livestock producers about the benefits of customized vaccines. These vaccines, created from pathogens isolated from specific animal populations, offer targeted disease control and reduce reliance on broad-spectrum antibiotics, aligning with global initiatives to combat antimicrobial resistance.
The market is anticipated to pick up steam in the future, with a predicted compound annual growth rate of 5.4%. Advances in vaccine research methods, rising demand for safer and more efficient animal health treatments, and regulatory frameworks that facilitate the use of autogenous vaccines in outbreak control are some of the main factors driving expansion. Autogenous vaccinations are in a strong position to significantly improve animal productivity and health if biosecurity measures are strengthened and animal husbandry methods are modernized.
Growth Drivers
Need for Targeted Immunization Drives Demand for Autogenous Vaccines
Commercial vaccines, while essential in animal disease prevention, present certain limitations that drive the demand for autogenous vaccines. According to the 2023 HealthforAnimals report, a significant portion of animal diseases continues to challenge veterinary medicine, with many traditional vaccines not always offering effective and timely responses to evolving threats. The majority of commercial vaccines rely on classical technologies, which dominate large portion of the market, but may not always be suitable for rapid response during localized outbreaks.
Many commercial vaccines are not designed to target specific strains that may vary across different herds or geographical regions. Autogenous vaccines offer a tailored approach by targeting specific pathogens involved in localized outbreaks. They are particularly valuable in scenarios where commercial vaccines are unavailable or insufficient.
With their potential to rapidly address evolving viral threats and support animal health, autogenous vaccines are becoming an increasingly important tool in modern veterinary medicine.
Market Restraining Factors
Stringent Regulatory Framework for Vaccine Production and Export
Stringent regulatory requirements remain a critical restraint for the autogenous vaccine market, impacting both vaccine development and commercialization.
The use of autogenous vaccines is typically restricted to specific herds or flocks, with strict limitations on the duration of use and mandatory retesting protocols. This narrow application scope limits scalability and may affect profitability for vaccine manufacturers. Additionally, compliance with regulatory requirements—such as batch-specific testing, stability assessments, and meticulous record-keeping—adds complexity to the manufacturing process. Smaller producers, in particular, may struggle to meet these stringent standards due to limited resources and infrastructure.
These regulatory constraints can also hinder the industry's ability to respond quickly to emerging animal health threats. While autogenous vaccines are designed to manage localized outbreaks effectively, compliance with strict guidelines can delay their deployment when rapid immunization is critical. This is particularly important in preventing the spread of zoonotic diseases that could pose risks to both animal and human health.
Key Market Opportunities
Technological Innovations Open New Avenues in Autogenous Vaccines
Technological advancements present a significant opportunity for the autogenous vaccine market, enabling more efficient and tailored solutions for animal health. Innovations in diagnostic technologies, vaccine formulation, and production processes have enhanced the speed and accuracy of autogenous vaccine development. These advancements help meet the rising demand for customized vaccines, especially in scenarios where commercial vaccines are not effective or unavailable.
Such advancements not only improve the safety and efficacy of autogenous vaccines but also support quicker vaccine production in response to emerging diseases. The integration of novel technologies like SRP® can lead to more predictable outcomes, reduce the risk of adverse reactions, and enable a more strategic approach to disease management in animal husbandry.
Strain Type Insights
Major Advantages of Bacterial Strains in Vaccines
The bacterial strain segment is projected to dominate the product category in the autogenous vaccine market, accounting for a significant 54.2% share in 2025.
Live bacterial strain vaccines provide several benefits, such as the ability to replicate a real infection, built-in adjuvant qualities, and better oral administration options. These reasons have led to a growing use of bacterial strains in the creation of specialized autogenous vaccines.
Animal Type Insights
High Disease Susceptibility Makes Poultry the Top Segment in Autogenous Vaccines
In the Autogenous Vaccine Market, poultry is the dominant animal type, holding the 30.1% market share in 2025. The high demand for autogenous vaccines in the poultry sector is primarily driven by the industry's scale, the susceptibility of poultry to various infectious diseases, and the need for tailored vaccines to manage localized outbreaks.
The frequent emergence of diseases such as avian influenza, Newcastle disease, and other bacterial infections in poultry farms necessitates customized immunization solutions, boosting the adoption of autogenous vaccines in this segment.
In 2023, the Animal and Plant Health Inspection Service (USDA), confirmed Highly Pathogenic Avian Influenza in commercial and backyard flocks, impacting over 378.5 million egg-laying chickens.
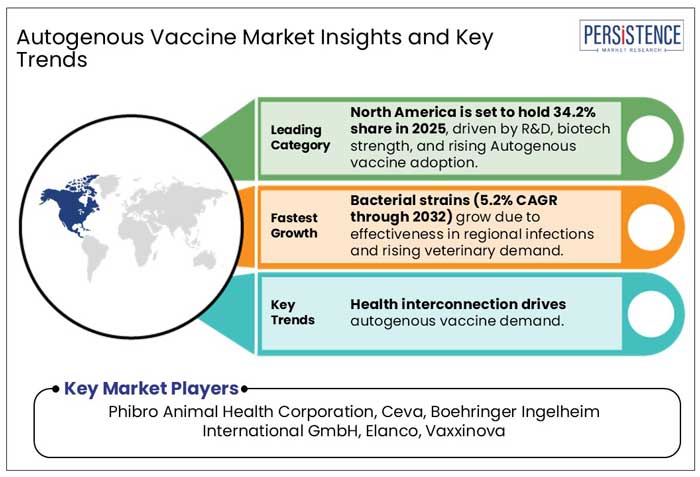
North America Autogenous Vaccine Market
Advanced Veterinary Practices Drive North America's Dominance in Autogenous Vaccines
North America is expected to account for 34.2% of the global autogenous vaccine market in 2025, driven by advanced veterinary infrastructure, strong regulatory support, and a growing emphasis on animal health. The region's robust poultry industry, coupled with the rising threat of emerging diseases, fuels the demand for tailored vaccine solutions.
Such developments highlight North America's leadership in leveraging autogenous vaccines.
Europe Autogenous Vaccine Market
Regulatory Strength and Industry Initiatives Propel Europe's Autogenous Vaccine Market
Europe is emerging as a significant hub in the autogenous vaccine market, expected to capture approximately 24.1% of the global market share by 2025. This growth is driven by a strong regulatory framework, evolving legislation, and proactive industry initiatives. The region's diverse legal landscape, as seen in countries like Germany and the UK, highlights both challenges and opportunities for autogenous vaccine manufacturers.
The ability to quickly produce and deploy autogenous vaccines during localized outbreaks, coupled with supportive legislative measures, reinforces Europe’s potential as a one of the major players in the global autogenous vaccine market.
Asia Pacific Autogenous Vaccine Market
Autogenous Vaccines Gain Traction Amid Livestock Challenges in Asia-Pacific
The Asia-Pacific (APAC) region is poised to capture a 20.2% share of the global autogenous vaccine market in 2025, supported by the region’s expanding livestock sector and rising demand for tailored disease management solutions.
Asia’s livestock sector significantly impacts forest ecosystems, with intensive farming practices driving demand for effective animal health measures. The need to manage diseases in densely populated livestock settings is accelerating the adoption of autogenous vaccines, positioning Asia Pacific as emerging player in the market.
The autogenous vaccine market features a competitive landscape marked by the presence of both global and regional players. Key companies such as Elanco Animal Health, Ceva Animal Health, Vaxxinova, Phibro Animal Health, and Boehringer Ingelheim International GmbH are actively investing in research and development to enhance their vaccine offerings. Collaborations, acquisitions, and innovations in vaccine technology are common strategies adopted by market participants to strengthen their foothold. The market's competitive dynamics are also influenced by evolving regulatory frameworks, technological advancements, and the growing need for tailored vaccines to address specific animal health challenges.
Key Industry Developments
|
Attribute |
Details |
|
Forecast Period |
2025-2032 |
|
Historical Data Available for |
2019 - 2024 |
|
Market Analysis Units |
Value: US$ Bn/Mn, Volume: As applicable |
|
Key Countries Covered |
|
|
Key Market Segments Covered |
|
|
Report Coverage |
|
|
Key Companies’ Profile |
|
|
Customization & Pricing |
Available upon Request |
Product:
By Animal Type
End User:
Region:
To know more about delivery timeline for this report Contact Sales

Increasing demand for customized veterinary vaccines for targeted disease prevention.
The market is projected to reach US$ 219.7 million by 2032.
They offer targeted immunization for specific pathogens when commercial vaccines fall short.
North America is expected to hold a 34.2% market share in 2025.
Poultry, with a projected 30.1% market share in 2025.