Comprehensive Snapshot of Swine Autogenous Vaccines Market Including Regional and Country Analysis in Brief.
Industry: Healthcare
Published Date: April-2025
Format: PPT*, PDF, EXCEL
Delivery Timelines: Contact Sales
Number of Pages: 150
Report ID: PMRREP35209
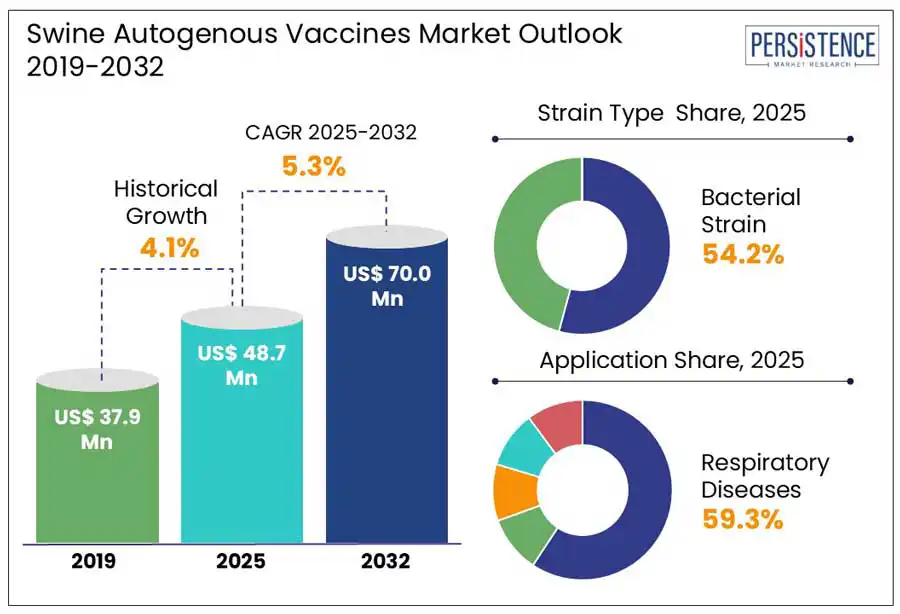
The global swine autogenous vaccines market is estimated to grow from US$ 48.7 million in 2025 to US$ 70.0 million to record a CAGR of 5.3% by 2032. According to Persistence Market Research, report, the rising prevalence of farm-specific and region-specific swine diseases that commercial vaccines fail to address effectively fuels the need. Autogenous vaccines developed using pathogens isolated directly from affected herds, offer a targeted disease control solution while supporting antibiotic reduction efforts in livestock farming.
The widespread adoption of vertically integrated swine production systems in North America streamlines disease surveillance and accelerates deployment of autogenous vaccines. Meanwhile, East Asia is emerging as a high-potential market where modernization efforts and improved biosecurity are encouraging the uptake of herd-specific vaccination strategies. These systems streamline disease surveillance, enable rapid diagnostics, and facilitate timely vaccine deployment, strengthening herd health and biosecurity. Additionally, regulatory support for customized vaccines, especially in the U.S. and Europe encourages a broader adoption.
Technological advancements are also reshaping the industry. Platforms like SEQUIVITY® by Merck Animal Health use RNA particle technology to develop herd-specific prescription vaccines for pathogens such as Influenza A Virus in Swine (IAV-S), Porcine Circovirus (PCV), and rotavirus. This innovation enables rapid, multivalent vaccine production without handling live pathogens, improving both safety and efficiency. Furthermore, collaborations between major industry players, including Boehringer Ingelheim and Ceva Animal Health, continue to improve vaccine accessibility, particularly in emerging markets. As demand for farm-specific disease management solutions grows, advancements in diagnostics and biosecurity measures are expected to drive further expansion of the swine autogenous vaccine market globally.
Key Industry Highlights:
|
Global Market Attribute |
Key Insights |
|
Swine Autogenous Vaccines Market Size (2025E) |
US$ 48.7 Mn |
|
Market Value Forecast (2032F) |
US$ 70.0 Mn |
|
Projected Growth (CAGR 2025 to 2032) |
5.3% |
|
Historical Market Growth (CAGR 2019 to 2024) |
4.1% |
The adoption of precision livestock farming (PLF) is transforming swine health management by enabling early disease detection, data-driven decision-making, and targeted autogenous vaccine use. AI-driven sensors, automated monitoring, and predictive analytics help identify pathogens early, reducing disease outbreaks and improving herd productivity. In February 2023, MACSO Technologies Limited deployed AI-powered sensors on a U.S. swine farm, cutting mortality rates to 1.4% in monitored barns compared to 5.7% in others, demonstrating the impact of real-time health monitoring.
Initiative such as the USDA’s Precision Geospatial & Sensor Technologies program are further advancing disease prevention by integrating precision diagnostics and biosecurity measures. These advancements enhance vaccine effectiveness, allowing swine producers to implement customized immunization strategies tailored to farm-specific disease risks. As PLF adoption grows, autogenous vaccines are becoming increasingly vital in modern swine health management, offering a proactive approach to disease control and herd protection.
A significant restraint in the swine autogenous vaccine market is the limited awareness and understanding among small and independent producers regarding the benefits and availability of customized vaccines. These farmers often rely on conventional commercial vaccines or traditional disease management practices due to limited exposure to newer, farm-specific solutions. For example, in several parts of Southeast Asia and Latin America, smallholder farms represent a large portion of pork production but typically lack access to veterinary consultations or the diagnostic support necessary for autogenous vaccine use. This knowledge gap hinders adoption despite the growing need for targeted disease control measures in densely stocked and resource-limited environments. Bridging this awareness gap through training programs, government initiatives, and veterinary outreach could be vital for expanding market reach.
Advancements in vaccine development technologies are driving new opportunities in the swine autogenous vaccine market, making these solutions more efficient, scalable, and accessible. Innovations such as RNA particle vaccines-exemplified by Merck’s SEQUIVITY® platform-allow for rapid, herd-specific vaccine development without handling live pathogens, significantly reducing production time. Additionally, protein subunit platforms like those developed by Medgene Labs, enhance vaccine stability and immune response ensuring safer and targeted disease prevention. These advancements enable faster adaptation to emerging swine diseases, improving herd health and biosecurity while expanding the global adoption of autogenous vaccine strategies.
The bacterial strain segment is projected to dominate accounting for a 54.2% share in 2025. Live bacterial vaccines mimic infections, enhance immune response, and allows oral administration, making them ideal for herd-specific disease control. Rising cases of Actinobacillus Pleuropneumonia and Streptococcus suis along with reduced antibiotic use drives demand for custom bacterial vaccines.
The viral strain segment is gaining traction with increasing demand for autogenous vaccines targeting Porcine Reproductive and Respiratory Syndrome (PRRS) and Influenza A, where commercial vaccines show limited efficacy.
In 2025, livestock farming companies are expected to dominate the swine autogenous vaccine market, accounting for approximately 63.2% of the total market share. This dominance is driven by the increasing need for farm-specific disease management, particularly in large-scale swine production systems. With the rising concerns over emerging disease threats and restrictions on antibiotic use, swine producers are turning to autogenous vaccines for tailored protection against localized pathogens. Moreover, the adoption of data-driven disease management strategies is further boosting the use of autogenous vaccines, allowing for more effective herd protection, and reduced economic losses.
While veterinary clinics and hospitals play a crucial role in diagnostics and vaccine administration, and veterinary research institutes contribute to vaccine development, livestock farming companies remain the primary end-users, as they directly benefit from customized vaccines to improve herd health and productivity.
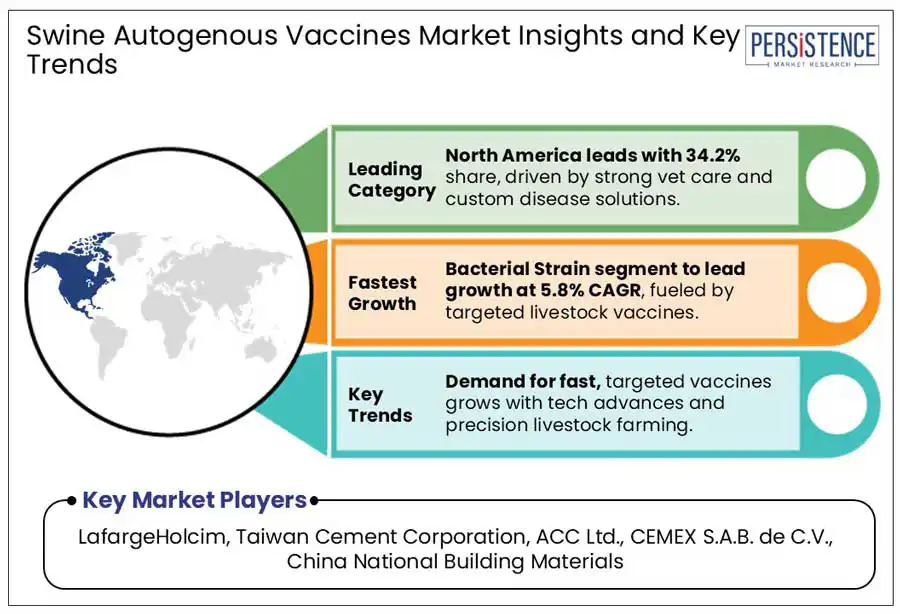
In 2025, North America is expected to hold 34.2% of the global swine autogenous vaccine market, with the U.S. leading the segment due to strong USDA regulatory oversight and an advanced veterinary infrastructure. The U.S. swine autogenous vaccine market is poised for steady growth, driven by rising disease threats, antibiotic restrictions, and advancements in precision livestock farming. In the early 2025, American Association of Swine Veterinarians (AASV) Industry Support Council aligned a collective discussion upon emerging disease threats, vaccine advancements, and biosecurity improvements with leading companies such as Merck Animal Health, Zoetis, and Ceva Animal Health.
By 2025, Europe is expected to account for 24.1% of the global swine autogenous vaccine market, driven by strong regulations and industry-led initiatives. In November 2024, the World Organization for Animal Health (WOAH) initiated a research publication on autogenous vaccines, following recommendations from the Second Global Conference on Antimicrobial Resistance, aiming to establish standardized guidelines as alternatives to antimicrobials. This regulatory advancement positions Europe as a key player in enhancing disease control strategies for swine herds, reinforcing its role in the global autogenous vaccine market.
East Asia swine autogenous vaccine market is set to capture a 17.2% global share in 2025 driven by the region’s expanding pork industry and growing demand for customized disease management. According to recent data published in 2022, China produced approximately 52 million tonnes of pork, representing 44.1% of global pork production, with the industry valued at around $150 billion. Frequent disease outbreaks, including African Swine Fever, have intensified the need for enhanced biosecurity and herd-specific vaccines. As both large-scale farms and small-scale operations adopt advanced health strategies, the demand for autogenous vaccines continue to grow across the region.
The global swine autogenous vaccine market is highly fragmented, with numerous manufacturers developing customized solutions for herd-specific disease management. The market encompasses a variety of vaccine formulations, including bacterial and viral strains, tailored to address emerging health threats in different regions. Advancements in diagnostics and vaccine production technologies are improving efficacy, accessibility, and adoption, driving market growth.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis Units |
Value: US$ Mn, Volume: As Applicable |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Strain Type
By Application
By End-user
By Region
To know more about delivery timeline for this report Contact Sales

The global swine autogenous vaccines market is estimated to increase from US$ 48.7 Mn in 2025 to US$ 70.0 Mn in 2032.
Rising farm-specific disease outbreaks, increasing restrictions on antibiotics, advancements in precision livestock farming, and the growing demand for customized disease management solutions.
The market is projected to record a CAGR of 5.3% during the forecast period from 2025 to 2032.
Opportunities in the swine autogenous vaccine market include increasing demand for personalized vaccine solutions to combat emerging infectious diseases and improve herd health management in the swine industry.
Key players in the swine autogenous vaccines market include Merck & Co., Inc., Ceva Animal Health, Phibro Animal Health, Vaxxinova, and Dopharma, among others.