North America Quadriplegia Care Devices Market Segmented By Stimulation Devices, Assistive Devices, Mobility Devices, Hospital Beds, Transfer Bench and Boards, Braces & Supports, Orthopaedic Splints Product with Incomplete, Complete Quadriplegia
Industry: Healthcare
Published Date: April-2022
Format: PPT*, PDF, EXCEL
Delivery Timelines: Contact Sales
Number of Pages: 166
Report ID: PMRREP33058
The North American quadriplegia care devices market was valued at US$ 414.5 Mn in 2021, with an estimated growth rate of 5.4% CAGR for the next ten years. As such, revenue from the sales of quadriplegia care devices in the region is predicted to reach US$ 741 Mn by 2031.
| Attribute | Key Insights |
|---|---|
|
North America Quadriplegia Care Devices Market Size (2020) |
US$ 414.5 Mn |
|
Estimated Market Value (2021) |
US$ 436.2 Mn |
|
Projected Market Value (2031) |
US$ 741 Mn |
|
Market Growth Rate (2021-2031) |
5.4% CAGR |
As projected by Persistence Market Research, hospital beds accounted for more than 27% market share in the North America quadriplegia care equipment market in the year 2020.
Overall demand for quadriplegia care devices in North America accounted for 15.9% share of the spinal cord trauma market, which was valued at US$ 2.7 Bn in 2020.
The North American market for quadriplegia care devices expanded at a CAGR of 4.8% over the last five years (2016-2020).
Rising prevalence of spinal cord injury necessitates medical items such as robotic arm systems, prosthetics and services based on usability.
Moreover, increasing number of trauma cases is another key factors promoting the growth of the North American quadriplegia treatment devices market.
Continuous innovations and technological advancements that address the needs of patients and users are expected to drive the quadriplegia care devices market in terms of sales revenue going forward.
For example, conventional braces and support systems, and also beds, lack some of the techniques, which have been currently fixed accordingly for patients’ needs and benefits. Hot and cold therapies provided by new braces continue to dominate conventional braces systems. This dual feature in orthopaedic braces is further expected to favour market expansion over the coming years.
The North America quadriplegia care systems market is expected to expand at a CAGR of 5.4% and record sales worth US$ 741 Mn by the end of 2031.
“Increasing Prevalence of Spinal Cord Injuries & Technological Advancements in Products”
Recent developments in research areas such as computer science, robotics, artificial intelligence, and sensor technology have broadened the range of possible applications that support disabled people. Powered wheelchairs have extended the capabilities of standard wheelchairs by introducing control and navigational intelligence.
Advanced quadriplegia devices are specifically intended to improve mobility with high efficiency, and limit stress-strain with flexibility in height. The quadriplegia care devices market in North America is creating huge opportunities for key players owing to the widespread acceptance and adoption of technologically-advanced devices driven by rigorous innovative research practices and earlier launches of products.
Additionally, there is lack of awareness of osteoporosis among. Few NGOs are working toward spreading awareness about osteoporosis and its diagnosis through quadriplegia care solutions.
This attempt by NGOs to raise awareness about osteoporosis can create huge demand for quadriplegia care devices in the market of North America.
“Regulations, High Cost of Material-based Braces, and Use of Second Hand Quadriplegia Devices”
Spinal braces used for supporting body weight are well-known for their durability and strength, and are designed using soft materials such as cotton/elastic blends, canvas, and neoprene. Back braces typically range from US$ 40 to US$ 1,000, depending on material type.
Material cost is an important factor for braces, but the high cost associated with braces might dent market growth in the future.
The FDA has a strong regulatory framework that needs to be followed by companies operating the market. Most new companies face quality and compliance failure issues with established regulatory guidelines. Any failure in GMP compliance leads to obstacles in market authorization. Hence, regulatory agencies are the major barriers to the entry of new comers into the quadriplegia care devices market.
Additionally, a number of quadriplegia care products are left behind each year by deceased patients. These products are often donated to hospitals and other charities or recycling groups. Used quadriplegia care devices can be resold in garage sales or refurbished goods stores. Hence, use of second hand quadriplegia care devices hampers the growth of the North American quadriplegia care devices market to some extent.
Why Do Quadriplegia Care Device Suppliers Need to Focus on the U.S.?
“High Incidence of Quadriplegia in the U.S.”
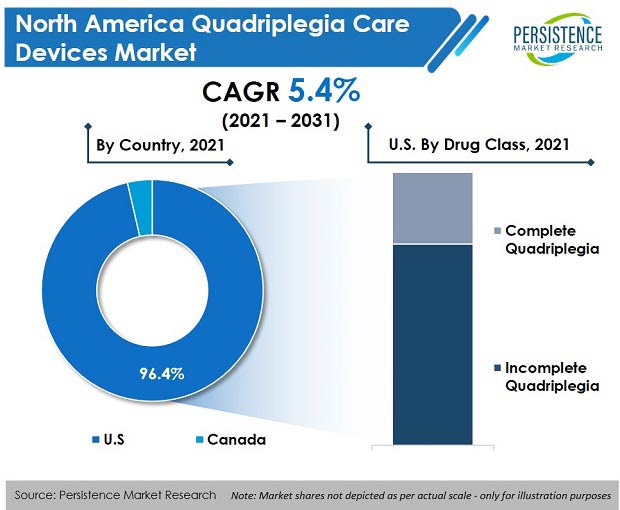
The U.S. quadriplegia care devices market dominated the North American region with a market share of more than 90% in 2020, and is projected to continue experiencing high growth throughout the forecast period.
The country’s large share is due to factors such as growing consolidation among healthcare providers, widespread adoption of quadriplegia care devices to reduce rising healthcare costs, and stringent regulations and guidelines imposed by government and non-government authorities.
Furthermore, the presence of a large number of competitors in the market is a major factor in the U.S.’s large share of the quadriplegia care devices market in North America.
What is the Outlook for the Canada Quadriplegia Care Devices Market?
“Increasing Incidence of Spinal Cord Injuries in Canada”
Canada accounted for 3.6% market share by value of the total North American quadriplegia care devices market, and was valued at US$ 15.6 Mn in 2020.
High prevalence of spinal cord injuries will drive consumption of quadriplegia treatment devices in the country.
Which Quadriplegia Care Product is High in Demand?
“High Demand for Hospital Beds across the Continent”
Hospital beds are driving the growth of the North American market for quadriplegia treatment devices, wherein, they accounted for 27.7 market share in the year 2020.
Growth of this segment is primarily attributed to the availability of technologically-advanced beds such as computer-controlled beds and self-rotating beds designed specifically for paraplegic and quadriplegic care.
Unique hospital beds for spinal cord injuries provide natural nocturnal motion of the body, improved quality of sleep, and reduce many health risks associated with spinal cord injuries.
Which Indication is Most Common in Quadriplegia Care?
“Sports Injuries & Car Accidents Most Common Cause of Incomplete Quadriplegia”
Incomplete quadriplegia accounted for 73.8% market share in the year 2020, and the segment is expected to expand at a CAGR of 5.1% over the forecast period.
Sports injuries and car accidents are the most common causes of incomplete quadriplegia, which can manifest themselves differently in each person. Some have mild lesions and are able to move and feel areas below their injury, while others suffer from more severe cases.
Strategic acquisition and agreement activities are being seen among key quadriplegia care device manufacturers. These collaborative activities incorporate companies allowing technology transfer and exchange of rights of sale, installation, training, servicing, and repairing services to regional players and third-party service vendors.
The market is dominated by players who have a strong product portfolio and patented technologies dedicated to the development of quadriplegia care devices, along with regional service and support facilities.
Some of the instances are:
Key players operating the North American quadriplegia care devices market are working toward partnerships to build on a leadership position in acute patient services with the addition of innovative devices.
| Attribute | Details |
|---|---|
|
Forecast Period |
2021-2031 |
|
Historical Data Available for |
2016-2020 |
|
Market Analysis |
US$ Million for Value |
|
Key Countries Covered |
|
|
Key Market Segments Covered |
|
|
Key Companies Profiled |
|
|
Report Coverage |
|
|
Customization & Pricing |
Available upon Request |
North America Quadriplegia Care Devices Market by Product:
North America Quadriplegia Care Devices Market by Indication:
North America Quadriplegia Care Devices Market by End User:
North America Quadriplegia Care Devices Market by Country:
To know more about delivery timeline for this report Contact Sales

The North American quadriplegia care devices market is currently valued at US$ 414.5 Mn, and is expected to grow 1.7X over the forecast period.
The North American market is set to witness a CAGR of 5.4% over the forecast period and be valued at US$ 741 Mn by 2031.
The North America quadriplegia care equipment market expanded at 4.8% CAGR from 2016 to 2020.
Increasing strategic acquisitions & collaborations, smartphone apps helping quadriplegic users with their disabilities, incorporation of hot and cold therapies, and emergence of advanced technology in functional electrical stimulation are keys trends shaping this industry.
Incomplete quadriplegia is driving high demand for quadriplegia care devices as it is commonly occurring, and held 73.8% market share in 2020.
The U.S. quadriplegia care devices market accounted for more than 96% share of the North American market in 2020.