Europe Precision Diagnostics Market
Industry: Healthcare
Published Date: November-2024
Format: PPT*, PDF, EXCEL
Delivery Timelines: Contact Sales
Number of Pages: 173
Report ID: PMRREP34894
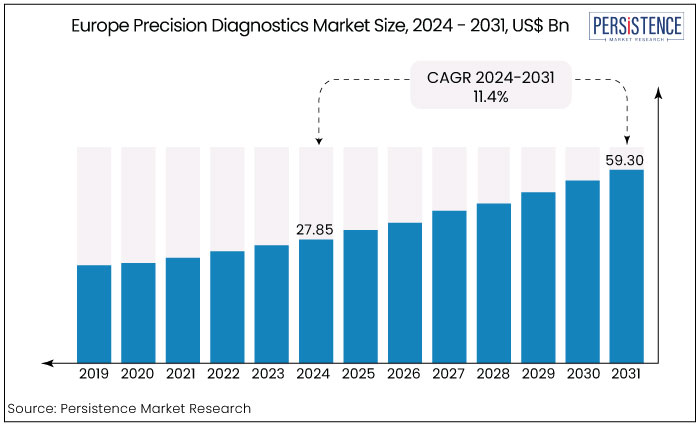
The Europe precision diagnostics market is estimated to rise from US$ 27.85 Bn in 2024 to US$ 59.30 Bn by 2031. The market is projected to record a CAGR of 11.4% during the forecast period from 2024 to 2031.
Government agencies in Europe are investing significantly in precision diagnostic labs to foster innovation and adoption of precision diagnostics, thereby augmenting the market. New-age technologies like Next-Generation Sequencing (NGS) are also likely to create novel opportunities. In December 2023, for instance, Deidentified Whole Genome Sequencing (WGS) data on about 500,000 individuals was made public by U.K. Biobank. This allowed researchers worldwide to examine the genomics findings in conjunction with the individuals' 15 years of medical records.
Key Highlights of the Market
|
Market Attributes |
Key Insights |
|
Europe Precision Diagnostics Market Size (2024E) |
US$ 27.85 Bn |
|
Projected Market Value (2031F) |
US$ 59.30 Bn |
|
Global Market Growth Rate (CAGR 2024 to 2031) |
11.4% |
|
Historical Market Growth Rate (CAGR 2019 to 2023) |
9.9% |
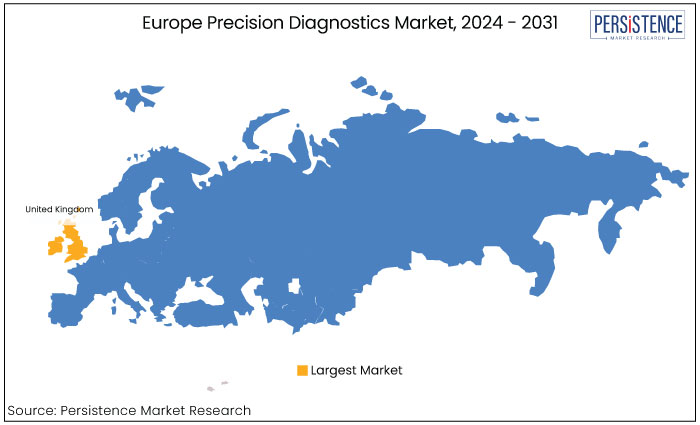
Persistent economic expansion is a key aspect driving demand in the U.K. Increasing government initiatives supporting the healthcare sector are further accelerating market expansion.
The U.K. is anticipated to generate the dominant market share in 2024 backed by rising number of research centers and laboratories. Enhancement of various genomic analysis techniques is another key factor augmenting demand in the country.
Genomic medicine pertains to cancer cases in which comprehending tumor cells enable physicians to provide precision therapy. This is expected to significantly accelerate the market in the U.K. In addition, emerging technologies like big data are likely to create new opportunities in the country’s pharmaceutical and biotechnology industries.
|
Category |
Market Share in 2024 |
|
Type- Genetic Tests |
55% |
Based on type, the market is segmented into genetic tests, direct to consumer tests, and esoteric tests. Among these, the genetic tests segment dominates with around 55% of Europe precision diagnostics market share in 2024. Increasing demand is attributed to enhanced comprehension of genetic problems, the emergence of customized medicine, and a favorable regulatory framework.
The segment has recently experienced substantial investments and partnerships among diagnostic firms, pharmaceutical corporations, and academic institutions. Such collaborations seek to create novel genetic tests and incorporate them into clinical practice, thereby augmenting market expansion. For instance,
|
Category |
Market Share in 2024 |
|
Application- Oncology |
26% |
Based on applications, the market is divided into oncology, respiratory diseases, skin diseases, and CNS disorders. Among, the oncology application segment dominates the market with a share of 26% in 2024. Growth is primarily attributed to the rising incidence of cancer and the need for early detection and surveillance of the disease.
As per the World Cancer Research Fund, in 2021, around 393,017 cases of cancer were reported in the U.K. alone. Out of these cases, nearly 193,076 were in females, whereas 199,941 were in males. This significant incidence rate necessitates the development of more tailored and precise diagnostic techniques to detect specific cancer types and their genetic profiles. It is likely to help enable targeted treatment strategies. Government investments in the field of oncology are also anticipated to enhance the segment throughout the projected period.
The Europe precision diagnostics market growth is projected to be driven by ongoing development of new medications with the assistance of genome therapies. In addition, rising emphasis on the expansion of healthcare centers with high-quality treatment procedures is likely to boost demand.
The market is further set to be significantly impacted by the increasing benefits of precision medicine in cancer diagnostics and therapeutics. Rising number of cancer cases and increasing demand for precise medications from patients are a couple of other factors boosting sales.
The market is also expected to surge due to the ongoing research and development activities to discover new genetic technologies. Increasing demand for next-generation sequencing techniques and rising number of clinical trials in Europe are estimated to create opportunities.
Precision diagnostics facilitate the expeditious delivery of treatment by offering a precisely timed and accurate explanation of a patient's health problems. The high efficiency and target-specific features of personalized medicine have led to innovations in genomics research and biomarkers. These are predicted to help healthcare professionals improve patient outcomes.
The Europe precision diagnostics market experienced a steady CAGR of 9.9% in the historical period between 2019 and 2023. This growth was mainly attributed to advancements in genomics, molecular diagnostics, and personalized medicine.
Increasing demand for early and accurate disease detection, especially for cancer, cardiovascular diseases, and rare genetic disorders, was a key factor contributing to expansion. Governments and healthcare institutions in Europe increasingly adopted precision diagnostics to improve patient outcomes. Advancements in Next-Generation Sequencing (NGS) and biomarker discovery further accelerated innovation. However, challenges like the high costs of precision diagnostic tools and limited infrastructure in a few areas restricted market growth.
The market is expected to witness a CAGR of 11.4% from 2024 to 2031. It is likely to be driven by ongoing technological innovations, including AI-driven diagnostic tools and cost-effective genomic solutions.
Increasing integration of precision diagnostics into routine healthcare practices, along with the rising demand for personalized treatment plans, is set to further fuel market expansion. Government initiatives supporting precision medicine research and regulatory approvals for novel diagnostic technologies are also expected to improve market accessibility.
As awareness of precision diagnostics rises among healthcare professionals and patients, adoption rates are likely to rise. Countries such as Germany, France, and the U.K. are anticipated to lead the market. This will likely be supported by rising collaborations between diagnostic companies and pharmaceutical firms to develop targeted therapies.
Urgent Need for Early Detection of Diseases to Augment Demand
One of the primary growth drivers for the Europe precision diagnostics market is the increasing prevalence of chronic diseases like cancer. As the population ages, conditions such as cardiovascular diseases, diabetes, and cancer are becoming more widespread, driving demand for accurate and early diagnosis.
Precision diagnostics allow for the identification of disease at a molecular level, which is essential for developing targeted treatment plans. This capability is especially vital in oncology, where detecting specific gene mutations can guide more effective and personalized therapies.
As healthcare providers prioritize early detection and preventive measures, the need for unique diagnostic tools will continue to surge, boosting the market. Europe’s healthcare system is hence emphasizing the improvement of patient outcomes and reduction of healthcare costs, thereby boosting adoption of precision diagnostics.
Emergence of Liquid Biopsy and Biomarker Discovery to Boost Growth
Technological innovations in genomics and molecular diagnostics are a significant driver of growth in the market. Innovations such as NGS, liquid biopsy, and advanced biomarker discovery have revolutionized how diseases are diagnosed, making the process highly accurate, quick, and less invasive.
NGS allows for detailed mapping of genetic information, leading to more personalized treatments based on a patient’s genetic profile. Liquid biopsies offer a non-invasive way to detect cancer and other diseases at an early stage, significantly improving survival rates.
The continuous development of molecular diagnostic technologies enables more precise identification of pathogens, genetic mutations, and disease markers. As these technologies become more affordable and widely adopted across healthcare systems, they are expected to contribute substantially to the regional market’s growth.
Difficulty for Small-scale Clinics to Adopt New Tools due to High Cost
One of the primary growth restraints is the high cost associated with advanced diagnostic technologies. Precision diagnostics, such as NGS, molecular diagnostics, and personalized testing, require sophisticated equipment, skilled professionals, and expensive reagents. These high costs can be prohibitive for healthcare providers, especially in countries with limited budgets or underfunded public health systems.
While large-scale medical institutions in wealthy countries like Germany, France, and the U.K. may afford these technologies, small or rural clinics across Europe may struggle to implement them. As a result, market penetration remains limited, with several patients not gaining access to these advanced diagnostic tools. Without significant cost reduction through innovation or government subsidies, the high prices will likely continue to act as a barrier to widespread adoption.
Regulatory Hurdles and Complex Approval Processes
Another key restraint is the complex regulatory environment of Europe. While the European Union (EU) has made efforts to standardize regulations across member countries, significant challenges remain in navigating the approval process for precision diagnostic products.
Each country may have different requirements for medical device approval. These stringent guidelines aimed at ensuring patient safety can lead to delays in bringing innovative diagnostics to market. For example,
The European Union’s in Vitro Diagnostic Regulation (IVDR), which came into effect in 2022, has imposed additional requirements for precision diagnostic devices. This made compliance more difficult and time-consuming for manufacturers.
Small-scale companies, in particular, may face financial and operational challenges in meeting these regulations. This could slow down innovation and reduce the number of new products entering the market. These regulatory barriers continue to be a critical factor limiting the market’s growth.
Companies Focus on Offering Affordable and Accessible Diagnostics Tools
One of the most transformative opportunities in the Europe precision diagnostics market lies in the growing focus on personalized medicine. Precision diagnostics are at the heart of personalized healthcare, allowing for treatments tailored to individual patients' genetic and molecular profiles.
As healthcare systems in Europe increasingly shift toward more personalized approaches, particularly in the treatment of cancer and genetic disorders, demand for precision diagnostics is set to soar. This opportunity is driven by advancements in technologies such as NGS and biomarker testing. These enable healthcare providers to identify specific genetic mutations or disease markers.
Ongoing expansion of personalized medicine will likely help improve patient outcomes by delivering more effective and targeted treatments. Additionally, it can reduce healthcare costs by minimizing trial-and-error prescribing and adverse drug reactions. Companies that can develop more affordable, accessible, and scalable precision diagnostic tools stand to gain significantly from this trend.
The Europe precision diagnostics industry is highly competitive, with a mix of established players and emerging local companies. Key players include Roche Diagnostics, Illumina, Thermo Fisher Scientific, and QIAGEN. All of these players dominate with advanced genomic technologies, molecular diagnostics, and strong research capabilities. These companies are expanding through strategic collaborations and product innovations.
Start-ups and small-scale firms, particularly those specializing in AI-driven diagnostics and personalized medicine, are also gaining traction. They are capitalizing on unmet needs in specific niches like rare diseases and oncology. Regional companies are increasingly forming partnerships with research institutions to enhance their product offerings, intensifying competition and innovation in the market.
Recent Industry Developments
|
Attributes |
Details |
|
Forecast Period |
2024 to 2031 |
|
Historical Data Available for |
2019 to 2023 |
|
Market Analysis |
US$ Billion for Value |
|
Key Countries Covered |
|
|
Key Market Segments Covered |
|
|
Key Companies Profiled in the Report |
|
|
Report Coverage |
|
|
Customization and Pricing |
Available upon request |
By Type
By Application
By End Use
By Country
To know more about delivery timeline for this report Contact Sales

It is a branch of precision medicine involving the management of a patient's healthcare model.
It will likely reach a value of US$ 59.30 Bn in 2031.
When the same sample yields consistent results from multiple analyses, the test is considered precise.
Precision medicine is primarily given to patients suffering from cancer.
Genomic medicine, personalized medicine, and individualized medicine are a few other names.