Industry: Healthcare
Published Date: November-2024
Format: PPT*, PDF, EXCEL
Delivery Timelines: Contact Sales
Number of Pages: 191
Report ID: PMRREP28264
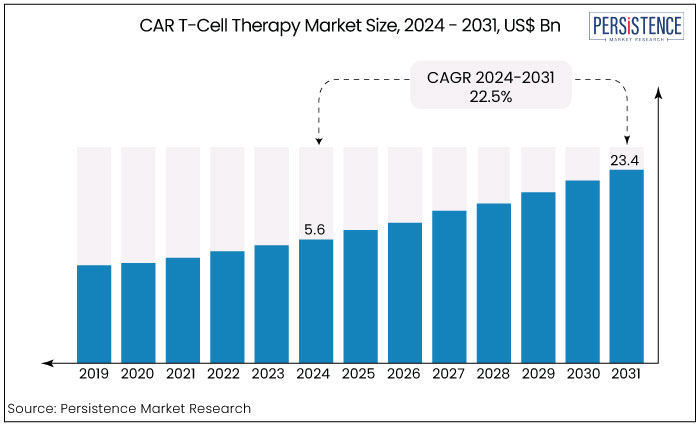
The global CAR T-cell therapy market is projected to witness a CAGR of 22.5% during the forecast period from 2024 to 2031. It is anticipated to increase from US$ 5.6 Bn recorded in 2024 to a staggering US$ 23.4 Bn by 2031.
The industry expansion is bolstered by presence of leading companies, investment in research and developments, and government funding. Increasing number of cancer rates and innovative treatment availability are fueling the demand for CAR T-cells further.
There was a 36.3% increase in new cancer cases during the period from 2000 to 2021, with annual diagnoses growing from around 1.3 million to 1.8 million. This number shows great rise in cancer incidence. The U.S. will see more than 2 million new cancer cases for the first time in 2024 according to the American Cancer Society projection highlighting the ongoing increase in cancer diagnoses. With the numbers rising, consumers are likely to adopt CAR T-cells therapies creating avenues for industry players.
Key Highlights of the Market
|
Market Attributes |
Key Insights |
|
CAR T-Cell Therapy Market Size (2024E) |
US$ 5.6 Bn |
|
Projected Market Value (2031F) |
US$ 23.4 Bn |
|
Global Market Growth Rate (CAGR 2024 to 2031) |
22.5% |
|
Historical Market Growth Rate (CAGR 2019 to 2023) |
11.6% |
|
Region |
Market Share in 2024 |
|
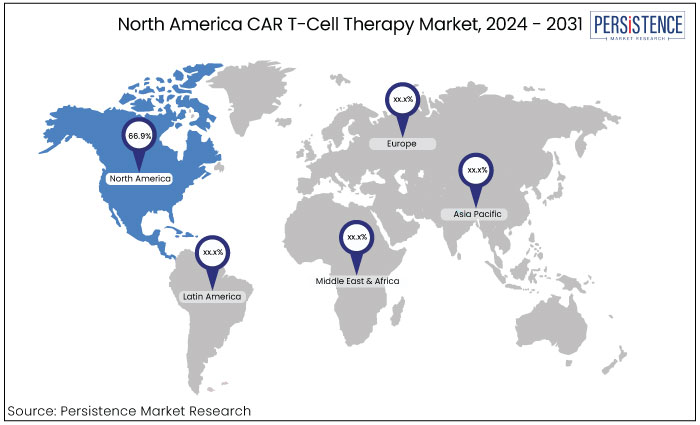
North America |
66.9% |
North America is expected to hold the 66.9% share of the market in 2024. This strong position is supported by the presence of key companies, increased government funding, and active research efforts. The comprehensive insurance coverage contributes to the region's dominance in the industry.
In 2024, the U.S. is expected to show over 98.8% of the value share within the North American CAR T-cell therapy market. The demand in the U.S. is growing due to an increasing number of life sciences companies focusing on developing and manufacturing CAR T-cells and enhanced quality control systems in the region. For example,
The U.S.-based company Boomi has launched a connector in October 2024. This device provides integration between third-party systems and the Veeva Vault platform, enhancing its collaboration with Veeva Systems for life sciences data sharing.
|
Region |
Market Share in 2024 |
|
Asia Pacific |
27.94% |
Asia Pacific is projected to be the significantly growing region in CAR T-cell therapy by 2031. It is anticipated to expand at a CAGR of 27.94% from 2024 to 2031. This rapid expansion is driven by a growing patient population and strong government support for products and market participants.
Leading companies are increasingly focused on developing advanced therapies, further boosting market growth. For example, China's the National Medical Products Administration (NMPA) has approved the supplemental biological license application for JW Therapeutics' Carteyva for adults with relapsed or refractory mantle cell lymphoma. This supplement is also supported by a Chinese clinical study.
Increasing number of clinical trials also contribute to industry expansion. According to the Clinical Trials Database 2023, around 103 CAR T therapies are currently in active clinical trials in China.
|
Category |
Market Share in 2024 |
|
Product - Yescarta (axicabtagene ciloleucel) |
57.8% |
Based on product, Yescarta (axicabtagene ciloleucel) is expected to reach a significant market share of over 57.8% in 2024. This therapy is specifically designed for patients with large B-cell lymphoma who have not responded well to traditional treatment options. Its strong performance in the market reflects its effectiveness in addressing the needs of patients facing this challenging condition.
A large randomized phase 3 clinical trial of CAR T-cell therapy Yescarta (axicabtagene ciloleucel) has found that it can help cure some people with aggressive non-Hodgkin lymphoma (NHL). The study presented at the American Society of Clinical Oncology (ASCO) annual meeting estimated that 55% of patients would still be alive four years after receiving axi-cel, compared to 46% of those who initially received standard treatment for relapsed disease. The results suggest axi-cel is now the preferred treatment for people whose diffuse large B-cell lymphoma has recurred quickly or proven resistant to standard initial treatment.
|
Category |
Market Share in 2024 |
|
Indication - Relapsed Large B-Cell Lymphoma |
90.7% |
Based on indication, relapsed large B-cell lymphoma is expected to account for around 90.7% of the market share in 2024. The high market share highlights the significant need for effective therapies in managing this challenging form of lymphoma, as patients often struggle with limited treatment options once their cancer returns or fails to improve.
Various companies and organizations are investing in studying and understanding large B-cell lymphoma and its cure. For example, American pharmaceutical and biotechnology company, Pfizer' Phase 3 ECHELON-3 study revealed that ADCETRIS, when combined with lenalidomide and rituximab, reduced patients' death risk by 37% compared to a placebo. The study results show a new hope for cancer treatment.
CAR T-cell therapy is an innovative treatment designed to combat cancer by modifying a patient's T-cells. It holds about 27.1% of the market share in the broader T-cell therapy landscape.
As cancer rates continue to rise, the demand for CAR T-cells is expected to increase significantly. In the U.S., from 2000 to 2021, the number of new cancer cases nationwide increased by nearly 36.3%. The country is expected to see around 611,000 cancer-related deaths, which translates to more than 1,600 deaths each day due to cancer. This increasing prevalence of cancer shows an acute need for an effective diagnosis like CAR T-cell therapy.
There is a notable surge in research and development within the life sciences and biotechnology fields focused on using chimeric antigen receptors for cancer treatment. Studies are being conducted worldwide to check the effectiveness of CAR T-cell therapy. This approach will enhance our understanding of its efficacy, mechanisms, and patient compliance, mainly for those battling leukemia and lymphoma.
The CAR T-cell therapy market has grown significantly during the period from 2019 to 2023. The increasing use of CAR T-cell therapy in cancer treatment remains a key factor fueled the market growth.
The increasing number of cancer cases in the U.S. drives the market forward. This process includes the extraction of T-cells from a patient and then sending them to the advanced manufacturing facility for further processing. Currently, there are only a few facilities that produce CAR T-cells in the U.S. and Europe.
Several manufacturers invested in CAR T-cell production due to the rising demand for cell-based therapies. After CAR T-cell therapy gained approval in 2017, major life sciences companies started to produce CAR T-cell products. Several companies are getting approval from the government to offer their products in the market. For example,
The CAR T-cell therapy market expansion will continue in the upcoming years. It is expected to reach a CAGR of 22.5% through 2031. The increasing number of clinical trials for CAR T-cells, key industry players in manufacturing, and the rising need for effective cancer treatments drive this growth.
CAR T-cell Therapy Demand to Skyrocket with Supportive Government and Awareness Programs
The key driver that fuels the CAR T-cell therapy market is the increasing prevalence of cancer. The availability of Patient Assistance Programs (PAPs) is another factor increasing the adoption of therapy.
Governments and organizations in developing initiatives and programs to create awareness about cancer. These approaches also help market to grow. For example, Organizations like the American Cancer Society and CancerCare offer a variety of programs aimed at helping patients navigate the challenges associated with cancer treatment.
This data shows the increasing need for effective solution like CAR T-cell and supportive government to create awareness about its importance.
Surge in Clinical Trials to Create New Avenue for Advanced Therapies
Another significant driver of growth in the CAR T-cell therapy market is the expansion of clinical trials. These trials aim at exploring new applications and improving existing therapies. Pharmaceutical companies and research institutions are investing in research to develop advanced CAR T-cell therapies to target various cancers and improve patient outcomes. This includes trials focused on enhancing the efficacy and safety profiles of CAR T-cells.
Studies investigating combination therapies that integrate CAR T-cell treatment with other modalities. The increasing number of clinical trials validates the potential of CAR T-cell therapies and attracts investment and interest from stakeholders. This contributes to further propelling market growth.
High Cost of CAR T-cell Therapy to Hamper Industry Growth
The high cost of CAR T-cell therapy is a key barrier to its widespread use and accessibility. One of the main reasons for this steep price is the complicated and personalized manufacturing process. Each treatment includes the extraction and genetic modifivation T-cells from the patient. This labor-intensive and time-consuming process leads to significant expenses. Some therapies like KYMRIAH and Yescarta often surpass $350,000 per treatment.
The report by the National Institute of Health shows that the CAR T-cell therapy is most expensive treatment and cost ranges from $373,000 to over $500,000 per patient. Due to this high cost, the market is facing changes in adoption worldwide.
Strict Eligibility Criteria of Therapy
Another challenge facing in the adoption of CAR T-cell therapy is available to patients who have already undergone extensive prior treatments. To be eligible for therapies like tisagenlecleucel or axicabtagene ciloleucel, patients must receive at least two lines of systemic therapies.
Some patients who have been through multiple therapies fear to tolerate the severe side effects of chimeric antigen receptor T-cell treatments. This limitation further lowers the adoption. As a result, strict eligibility criteria for CAR T-cell therapy is associated with higher treatment costs. This factor is likely to hinder the adoption of CAR T therapy.
Research and Development in Cancer Therapies to Create Growth Opportunities
A growing focus on research and development for cancer therapies like lung cancer, colorectal cancer, multiple myeloma, and prostate cancer are increasing. This trend is expected to create several growth opportunities for providers of CAR T-cell therapy. Strategic acquisitions can also help manufacturers accelerate their efforts to capture a larger share of the market.
Supportive government policies and funding for life sciences are expected to drive demand for CAR T-cell therapy in the near future. For example, In the U.S., the Life Sciences legislation allocates $1 billion over ten years to support various initiatives.
The increasing need for innovative cancer treatment options is anticipated to create significant revenue opportunities for those involved in the chimeric immunoreceptor therapy market.
Advancements in Therapies to Accelerate Industry Expansion
Use into solid tumors shows key opportunity for the CAR T-cell therapy market, allowing growth and advancements in treatment.
While CAR T-cell therapies have had great success in treating blood cancers like B-cell lymphomas and Acute Lymphoblastic Leukemia (ALL), their use in solid tumors has been more challenging. This is mainly due to unique obstacles such as the tough tumor microenvironment, the diversity of tumor cells, and physical barriers within solid tumors. Overcoming these challenges could lead to new opportunities for growth and innovation in the market. Companies in this field are developing several products and innovative therapies. For example,
The global CAR T-cell therapy market is growing continuously owing to the presence of several leading companies. These key players are focusing on introducing innovative therapies and treatment options.
Companies involved in CAR T-cell therapy are actively working to enhance their market presence through collaborations, regulatory approvals, acquisitions, and partnerships with both established and emerging players in the industry.
Governments from several countries also contributing to create awareness and funding for developments of these treatments. In 2020, the FDA approved a CAR T-cell therapy known as brexucabtagene autoleucel (Tecartus) for patients with mantle cell lymphoma.
Recent Industry Developments
|
Attributes |
Details |
|
Forecast Period |
2024 to 2031 |
|
Historical Data Available for |
2019 to 2023 |
|
Market Analysis |
US$ Billion for Value |
|
Key Regions Covered |
|
|
Key Market Segments Covered |
|
|
Key Companies Profiled in the Report |
|
|
Report Coverage |
|
|
Customization & Pricing |
Available upon request |
By Product
By Indication
By End User
By Region
To know more about delivery timeline for this report Contact Sales

Yes, the market is expected to reach US$ 23.4 Bn by 2031.
Novartis is one of the leading companies that make CAR-T cell therapy for B-cell malignancies.
China is the best country for CAR-T cell therapy as it has the highest number of CAR-T cell clinical trials.
Yes. CAR T-cell therapy is available in India.
Yes. CAR T-cell therapy is expensive.