Biosimilars Insulin Market Segmented By Insulin Glargine, Insulin Analog Product in Hospital Pharmacies, Retail Pharmacies Distribution Channel
Industry: Healthcare
Published Date: July-2022
Format: PPT*, PDF, EXCEL
Delivery Timelines: Contact Sales
Number of Pages: 250
Report ID: PMRREP11674
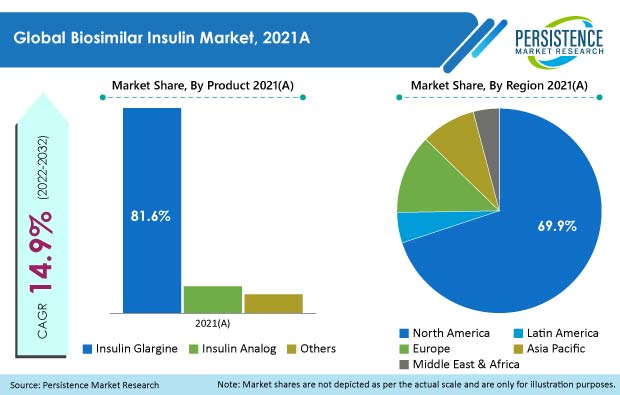
Revenue from the global biosimilar insulin market reached US$ 1.3 Bn at the end of 2021, with the global market estimated to surge ahead at a value CAGR of 14.9% to reach US$ 5.9 Bn by the end of 2032.
As assessed by Persistence Market Research biosimilar insulin sales accounted for approximately 6.7% revenue share in the global insulin market in 2021.
| Attribute | Key Insights |
|---|---|
|
Biosimilar Insulin Market Size (2022) |
US$ 1.3 Bn |
|
Projected Market Value (2032) |
US$ 5.9 Bn |
|
Global Market Growth Rate (2022-2032) |
14.9% CAGR |
|
Market Share of Top 3 Countries |
72.2% |
The sudden rise in the number of diabetic patients has made it difficult for healthcare professionals to provide biological therapy for insulin. The present focus lies in driving the cost down for the manufacturing of biosimilar insulin, which will accelerate the growth of large molecule biological products.
These special products have a favorable impact on the worldwide market, although the regulatory scenario in the case of biosimilar insulin is critical.
Biosimilar insulin went through lots of trials and failures and was not approved for a long time. Some of the weaknesses identified in the study reports included statistical errors, unclear calculations (a statistical analysis plan was not provided), and inconsistent or missing information.
Regulatory bodies such as the FDA and European regulatory agencies established relatively clear guidelines for the market approval of biosimilar insulin.
Manufacturers, with their research and development teams, started working on innovative products, and as the price of insulin drugs increased with time, the chance of entry of reference products and biosimilar insulin increased.
Regulatory agencies tried to deal with three linked issues in 2021 - decreasing anti-competitive behavior, lowering drug prices, and providing additional assistance in clarification of the regulatory pathway for a biosimilar.
A new race has been initiated between biosimilar and branded drug manufacturers. Demand has increased due to several factors such as the ongoing need for cost-effective drugs, recent approvals, and also the increased prevalence of diabetes.
The global biosimilar insulin market is thus likely to exhibit high growth over the coming years a value CAGR of 14.9% and reach a global market size of US$ 5.9 Bn by 2032.
“Cost-Effective Biosimilar Insulin – Need of the Hour”
Access to affordable insulin has become much more challenging for diabetics, particularly those in the low-income group. In several jurisdictions and through some commercial insurance companies, there have been recent improvements in lowering or capping the price of insulin.
Biosimilar insulin is being developed by companies such as Biocon, Eli Lilly and Company, Sanofi, BGP Pharma, Aspen, and Mylan (Viatris)
Such collaborations are expected to result in high adoption rates, which will drive market growth over the forecast period.
To bring more cost-effective and affordable technologies, manufacturers are also allocating funds to research and development. New technologies will soon be introduced as a result of increased research and development efforts by important organizations. These elements are anticipated to increase the acceptance and use of biosimilar insulin, culminating in market expansion over the coming years.
“Decreasing Prices of Standard Drugs”
By introducing novel products, the pharmaceutical business is experiencing exponential growth. Good manufacturing practices are being used more frequently to ensure the integrity of finished products. For continued supply, these items must adhere to very stringent regulatory requirements.
Biosimilar products already in use typically have initial list prices that are 15% to 35% lower than the list prices of reference products. If pharmaceutical industries decide to decrease the prices of standard drugs owing to the upcoming competitive pressure, it can decrease the demand for biosimilar insulin as the availability crisis will be fulfilled, and manufacturers can easily launch products with new prices respective to that of reference products.
The presence of standard drugs can attract more buyers, and hence, biosimilar products need to be as efficient and reliable as standard drugs.
Why is the U.S. Biosimilar Insulin Market So Prominent?
“Increasing U.S. FDA Approvals of Cold Storage Drugs”
The U.S. accounted for around 62.6% market share in the global biosimilar insulin market in 2021, and a similar trend is expected over the coming years.
Insulin glargine is a long-acting human insulin analog used to improve glycaemic control in adults, children, and adolescents with type 1 diabetes.
Similar to generic medications, Semglee can be automatically substituted by pharmacists for the reference product Lantus, without a clinician's approval. As a result of these approvals, demand for biosimilar insulin has increased in the U.S., which has raised the market's overall value for the drug.
Will the U.K Be a Lucrative Market for Biosimilar Insulin Products?
“U.K. Biopharma Industry Driving Demand for Biosimilar Insulin”
The U.K. held around 21.4% market share of the Europe biosimilar insulin market in 2021.
The United Kingdom is still the second-largest producer of active biotherapeutic medicines in the European Union. In the U.K. market, several biosimilar insulin products are becoming accessible. The first is the insulin glargine biosimilar Lantus®, which is the original brand and Abasaglar® is the biosimilar brand.
These products must always be prescribed by brand name because they are not interchangeable. Since they are less expensive than original medicines, biosimilar insulin offers the National Health Service (NHS) opportunities in terms of availability and healthcare costs.
Multinational corporations (MNCs), small- and medium-sized pharmaceutical companies, and small- and medium-sized biotechnology companies (SMEs) are all contributing to new advances in the nation, either together or independently. To ensure product quality as the industry expands, pharmaceutical companies such as Sanofi continue to go outside of conventional markets for industrial, identifiable, secured, and compatible supply chains that include biosimilar insulin.
How is India Emerging as a Prominent Market for Biosimilar Insulin?
“Biotech R&D Sector in India Focusing on Investments & Expansion for Product Launches”
India held the largest market share of 80.1% of the APAC biosimilar insulin market in 2021.
Expansion of the biopharmaceutical industry is one of the nation's key priorities as the Indian government strives for self-sufficiency. For instance, the Indian government is spending billions on biotechnology research and development. The government intends to spend even more this year, at 2.5 percent of GDP, according to official estimates, which show that scientific R&D spending hit US$ 291 billion in 2018, or slightly more than 2 percent of the GDP.
There are currently over 100 Indian biopharmaceutical companies engaged in the production and marketing of biosimilars. India has great potential and big aspirations and Indian biopharmaceutical businesses are modernizing their technology to enhance the capabilities of their workforce.
Which Product is Driving High Biosimilar Insulin Market Growth?
“High Demand for Development & Manufacturing of Biosimilars across Regions”
The insulin glargine products segment held around 81.6% share of the total biosimilar insulin market in 2021.
Factors such as the growing number of biotechnology and life science companies in developed regions, growing demand for the development and manufacturing of biological products, and increasing prevalence of type I diabetes are a few reasons that are expected to drive demand growth for insulin biosimilars, thus accelerating market growth.
In comparison to comparable therapies, insulin glargine was linked with stronger glycemic control in both, young and middle-aged T2DM patients.
Which Distribution Channel Accounts for Higher Sales of Biosimilar Insulin?
“Biosimilar Insulin Product Sales High through Retail Pharmacies”
Retail pharmacies held a high share of 46.7% with a market value of around US$ 597 Mn in 2021.
Retail pharmacies are well-positioned to respond to customer demand and provide biosimilar insulin. The result seen is improved scalability and uniformity after a drug is authorized for use in the international market.
Leading manufacturers are lowering the price of biosimilar insulin products to increase their market share globally. In the same way, several significant market competitors are seeking licensing and approvals. The growth of corporate partnerships to advance biosimilar insulin products is another key market trend being observed.
For instance:
| Attribute | Details |
|---|---|
|
Forecast Period |
2022-2032 |
|
Historical Data Available for |
2017-2021 |
|
Market Analysis |
USD Million for Value |
|
Key Countries Covered |
|
|
Key Market Segments Covered |
|
|
Key Companies Profiled |
|
|
Report Coverage |
|
|
Customization & Pricing |
Available upon Request |
Biosimilar Insulin Market by Product:
Biosimilar Insulin Market by Distribution Channel:
Biosimilar Insulin Market by Region:
To know more about delivery timeline for this report Contact Sales

The global biosimilar insulin market is currently valued at US$ 1.3 Bn.
Sales of biosimilar insulin are set to surge at 14.9% CAGR and reach US$ 5.9 Bn by 2032.
The U.S., U.K., and India drive most demand for biosimilar insulin, currently holding 72.2% market share.
The U.S. accounted for around 89.5% share of the North American market in 2021.
Biocon, Eli Lilly and Company, and Sanofi are the top three suppliers of biosimilar insulin products.
India held a share of 6.9% in the global biosimilar insulin market in 2021, and a regional share of 80.1% in APAC.
Retail pharmacies accounted for 46.7% market share in 2021.
The Middle East & Africa held 4.1% of the global market share in 2021.