Industry: Healthcare
Published Date: September-2022
Format: PPT*, PDF, EXCEL
Delivery Timelines: Contact Sales
Number of Pages: 250
Report ID: PMRREP2782
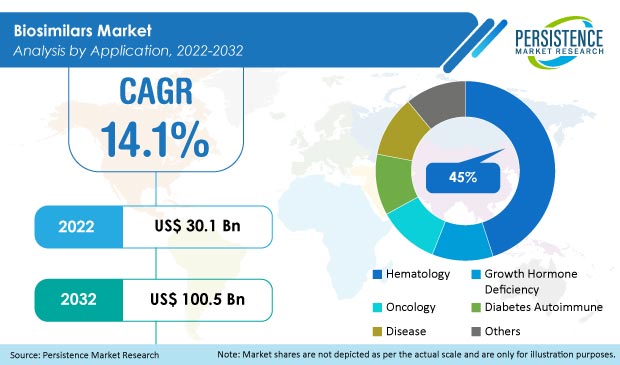
The global market for biosimilars is projected to expand at a noteworthy CAGR of 14.1% and reach a size of US$ 100.5 Bn by the end of 2032, up from the current industry value of 39.1 Bn.
The hematology vertical, based on application, accounts for almost 45% of the total share of market revenue after having been valued at US$ 34.3 Bn at the end of 2023.
| Report Attribute | Details |
|---|---|
|
Biosimilars Market Size (2024E) |
US$ 39.1 Bn |
|
Projected Market Value (2032F) |
US$ 100.5 Bn |
|
Global Market Growth Rate (2024-2032) |
14.1% CAGR |
|
U.S. Market Value (2032) |
US$ 21 Bn |
“Growing Adoption of Biosimilars in Healthcare Industry”
Biosimilars are being adopted at an increased rate owing to growing awareness about them. These are widely used to prevent and treat various chronic diseases such as cancer, diabetes, autoimmune diseases, CVDs, kidney failure, rheumatoid arthritis, hematological diseases, growth hormone deficiency, and infectious diseases. These growing healthcare issues are fueling the demand for biosimilars and will help the market gain momentum over the coming years.
Preference for biosimilars over traditional biologics is gaining traction in the market across the world. This trend is attributed to the economic nature of these solutions as compared to their parent biologics drugs. In addition, rapid increase in the population of geriatric people is also estimated to drive the demand for biosimilars in the years ahead.
“Increased Organizational Initiatives to Stimulate Biosimilar Adoption”
Numerous private and government bodies are taking initiatives to promote the use of biosimilars instead of synthetic drug products and conventional biologics. These initiatives are anticipated to fuel revenue generation opportunities for biosimilar makers during the forecast period.
Rapid growth of the pharmaceutical realm and higher prices of existing biological drugs are likely to foster attractive growth avenues in the biosimilars market.
“High Manufacturing Cost - A Market Dampener”
The production cost of biosimilars is comparatively higher and remains a roadblock to producing them on a massive scale. Along with this, the complexity involved in the production process is also expected to restrict growth impetuses in the market.
The easy availability of several affordable generic drugs is anticipated to limit the sales of biosimilars. One noticeable hurdle for market players is the establishment of biosimilarity through extensive analytical characterization.
Before undergoing the procedure to get regulatory approval, each biosimilar needs to face scrutiny by respective regulatory bodies to ensure similarity based on efficacy and safety. Moreover, resultant price competitiveness and price reductions in the industry are likely to bring challenges to the commercialization of biosimilars.
Discounted biosimilar prices will continue to hold a major impact on overall biosimilar sales, as patients will remain the key beneficiaries. Innovators’ price dropping with heavy discounts is a recent, ongoing trend among manufacturers based in progressing biosimilar markets. This will be a popular trend throughout the forecast period as well, eventually spurring the global biosimilars market growth.
Furthermore, biosimilar manufacturers are likely to trial multiple production volumes or production on different scales. The potential profitability of manufacturing multiple biosimilar products in the same facility can also be a favorable growth opportunity for biosimilar manufacturing companies during the forecast period.
A number of leading manufacturers are focusing on strategic collaborations. In addition, a large number of players are increasingly outsourcing the production of biosimilar products. Both these trends will favor the market over the next few years.
The global biosimilars market can be segmented based on product type, application, and distribution channel.
Geographical segmentation of the global biosimilars market is North America, Latin America, Europe, South Asia and Pacific, East Asia, and the Middle East and Africa.
The biosimilars industry was solely concentrated in the European market since 2006 when the first biosimilar was approved in the EU by the EMA (European Medicines Agency). Europe has been the first and the largest market for biosimilars, now followed by North America.
Owing to the ready availability of skilled labor, relatively lower manufacturing costs, and less complicated regulatory measures, the market for biosimilars in Asian countries is expected to witness significant growth in the near future. The biosimilars market is currently witnessing active growth in regions, such as China, India, and Korea. The number of biosimilar manufacturers within China is drastically surging, as a result of which the FDA of China recently developed and finalized the biosimilars guidelines confined to the market for biosimilars production, sale, and consumption across China.
Some of the leading Asian manufacturers are already trading approved biosimilar products in the EU. Several Asian market players are likely to get product approvals in Western markets over the forecast period.
Pfizer Inc., Eli Lily & Company, Sandoz International GMBH, Hospira Inc., Actavis, Inc., Biocon Ltd., Amgen, Inc., Teva Pharmaceutical Industries Ltd., Cipla Ltd, Dr. Reddy’s Laboratories Ltd., Stada Arzneimittel Ag, Celltrion, Inc., Wockhardt Ltd, and Mylan, Inc. are some of the key players profiled in PMR’s global biosimilars market report.
|
Attribute |
Details |
|---|---|
|
Forecast period |
2024-2032 |
|
Historical data available for |
2019-2023 |
|
Market analysis |
USD million for value |
|
Key regions covered |
|
|
Key countries covered |
|
|
Key market segments covered |
|
|
Key companies profiled |
|
|
Report Coverage |
|
|
Customization & pricing |
Available upon request |
By Product Type:
By Application:
By Distribution Channel:
By Region:
To know more about delivery timeline for this report Contact Sales

The global biosimilars market is currently valued at US$ 30.1 Bn.
Hamatology holds a major share in the biosimilars market.
The Asian region dominates the global market due to the easy availability of skilled labor, less complicated regulatory measures, and relatively lower manufacturing costs.
Global demand for biosimilars is expected to surge at 14.1% CAGR through 2032.
The U.S. is expected to account for around one-fifth of the global market share by 2032.