Industry: Healthcare
Published Date: July-2024
Format: PPT*, PDF, EXCEL
Delivery Timelines: Contact Sales
Number of Pages: 244
Report ID: PMRREP34618
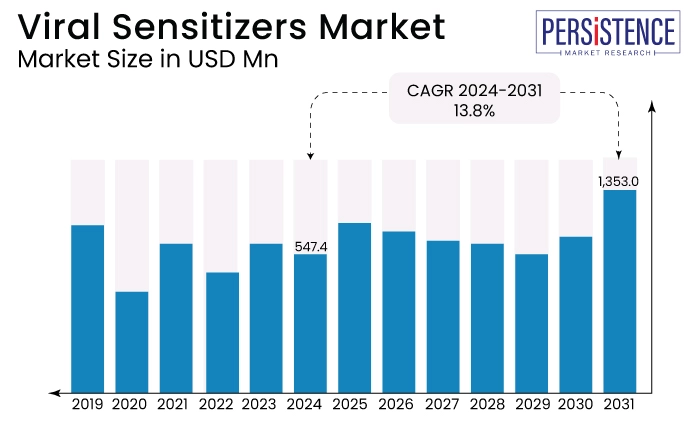
The global market is estimated to value at US$1,353.0 Mn by the end of 2031 from US$547.4 Mn estimated to be recorded in 2024. The market is expected to secure a CAGR of 13.8% in the forthcoming years from 2024 to 2031.
Key Highlights of the Market
|
Market Attributes |
Key Insights |
|
Global Viral Sensitizers Market Size (2024E) |
US$547.4 Mn |
|
Projected Market Value (2031F) |
US$1,353.0 Mn |
|
Forecast Value CAGR 2024 - 2031 |
13.8% |
|
Historical Value CAGR 2019 - 2023 |
12.5% |
The viral sensitizers market is an emerging sector within the biotechnology and pharmaceutical industries, focusing on agents that enhance the efficacy of viral treatments.
The sensitizers are crucial in optimizing therapies such as oncolytic virotherapy, which uses viruses to target and destroy cancer cells, and gene therapy, where they improve the delivery and expression of therapeutic genes.
The market is witnessing significant growth driven by advancements in biotechnology, increased investment in cancer research, and the rising prevalence of viral diseases.
Additionally, there is a growing trend towards personalized medicine, where sensitizers are tailored to individual patient profiles to maximize treatment outcomes. With ongoing R&D, the market growth is expected to accelerate, offering new opportunities for innovation and improved therapeutic strategies.
The market has experienced notable growth over the past decade, driven by advancements in biotechnology and an increasing understanding of viral mechanisms and their therapeutic potentials. Initially, the concept was met with scepticism due to the complexity of viral interactions within the human body.
However, breakthroughs in molecular biology and virology have paved the way for the development of more effective and safer sensitizers. Early research focused primarily on enhancing the efficacy of oncolytic viruses in cancer treatment, leading to a surge in clinical trials and subsequent approvals of new therapies.
As the understanding deepened, their applications expanded beyond oncology to include various infectious diseases and genetic disorders.
Looking ahead, the market is poised for substantial growth. The increasing incidence of chronic diseases, particularly cancer, continues to drive demand for more effective treatment options.
Personalized medicine is set to play a crucial role, with viral sensitizers being tailored to individual genetic profiles, thereby improving treatment outcomes and minimizing side effects.
Moreover, the ongoing COVID-19 pandemic has underscored the importance of viral research and accelerated the development of novel antiviral therapies, including sensitizers.
The market is also expected to benefit from technological advancements in drug delivery systems, enabling more efficient and targeted delivery.
As research continues to uncover new mechanisms of action and potential applications, the scope will likely expand further, encompassing a broader range of diseases and therapeutic areas.
Advances in Biotechnology and Genomics
The advent of precision gene editing technologies, particularly CRISPR-Cas9, has revolutionized the development of viral sensitizers. CRISPR-Cas9 allows for the precise modification of viral vectors, enhancing their ability to target specific cells and tissues with minimal off-target effects.
The precision ensures that these sensitizers can be tailored to interact with specific genetic markers or disease states, thereby increasing their therapeutic efficacy and safety.
The ability to fine-tune viral vectors at a genetic level means that treatments can be customized to individual patient profiles, paving the way for more effective personalized therapies.
The advances in biotechnology and genomics medicine are expanding the therapeutic applications beyond traditional areas. Initially focused on oncology, viral sensitizers are now being explored for a range of applications, including the treatment of genetic disorders, infectious diseases, and autoimmune conditions.
The ability to engineer viral sensitizers for specific therapeutic targets means that these agents can be adapted to address various medical needs, unlocking new market opportunities.
As research continues to unveil new possibilities, the scope will likely broaden further, solidifying their role in modern medicine.
Advances in biotechnology and genomics are key drivers propelling the market forward. These technological innovations enable the precise engineering, customization, and optimization, enhancing their therapeutic efficacy and safety.
As research and development processes become more efficient, and the range of therapeutic applications expands, the viral sensitizers market is poised for significant growth, offering new and improved treatment options for a variety of diseases.
Technical Complexity, and Manufacturing Challenges
Technical complexity and manufacturing challenges pose significant hurdles in the viral sensitizers market. The production involves intricate biotechnological processes that demand specialized equipment and expertise. These processes include the precise engineering of viral vectors, ensuring their stability, and maintaining their efficacy and safety profiles.
Scaling up production while maintaining consistency and quality standards is particularly challenging due to the sensitivity of viral vectors to environmental conditions and the risk of contamination during manufacturing.
The complexities not only increase production costs but also extend development timelines, potentially delaying market entry and limiting the availability of advanced therapies.
Moreover, ensuring regulatory compliance adds another layer of complexity. Regulatory bodies require rigorous testing and validation of manufacturing processes to ensure product safety and efficacy. Meeting these stringent regulatory standards further contributes to the technical challenges faced by manufacturers of viral sensitizers.
Addressing these complexities requires ongoing investment in research, infrastructure, and technological innovation to streamline manufacturing processes and accelerate the translation of promising therapies from development to market availability.
Increasing Adoption of Combination Therapies
The increasing adoption of combination therapies represents a pivotal opportunity in the viral sensitizers market. By combining viral sensitizers with complementary therapeutic modalities such as immunotherapy, chemotherapy, or targeted therapies, companies can potentially enhance treatment outcomes synergistically.
Such combinations leverage the unique mechanisms of viral sensitizers to amplify the effects of other treatments, overcome resistance mechanisms, and reduce the likelihood of disease recurrence.
This approach not only expands the therapeutic applications across various diseases but also addresses the complex challenges of treating heterogeneous conditions with multifaceted treatment strategies. Moreover, the trend towards combination therapies underscores a shift towards more personalized and tailored treatment approaches in healthcare.
Companies that successfully develop and commercialize combination therapies can differentiate their products in a competitive market landscape, potentially capturing greater market share and meeting the evolving needs of healthcare providers and patients seeking more effective and comprehensive treatment options.
|
Category |
Projected CAGR through 2031 |
|
Application - Oncolytic Viral Therapies |
14.5% |
|
End User - Pharmaceutical Companies |
14.0% |
Oncolytic Viral Therapies Dominant Area of Application
Pharmaceutical companies are typically the leading end users in the viral sensitizers market. These companies are heavily involved in the research, development, and commercialization of viral sensitizers for therapeutic applications. They have the resources and infrastructure to conduct extensive clinical trials, navigate regulatory processes, and bring products to market.
Pharmaceutical companies also collaborate with biotechnology firms, research institutes, and other stakeholders to advance viral sensitizer technologies and expand their therapeutic applications. Their leadership in the market is driven by their ability to invest in innovative treatments and leverage their global reach to deliver viral sensitizers to patients worldwide.
Pharmaceutical Companies Lead by End User
Pharmaceutical companies possess significant research and development capabilities, allowing them to invest in and advance viral sensitizers from early-stage discovery through clinical trials to market approval. They have the expertise to conduct extensive preclinical and clinical studies necessary to demonstrate safety, efficacy, and potential therapeutic benefits of viral sensitizers.
Pharmaceutical companies have experience navigating complex regulatory pathways and obtaining approvals from regulatory agencies such as the FDA (Food and Drug Administration) in the United States, or the EMA (European Medicines Agency) in Europe. They understand the stringent requirements for drug approval and have the resources to meet regulatory standards, ensuring compliance throughout the development and commercialization process.
|
Region |
Projected CAGR through 2031 |
|
North America |
14.7% |
|
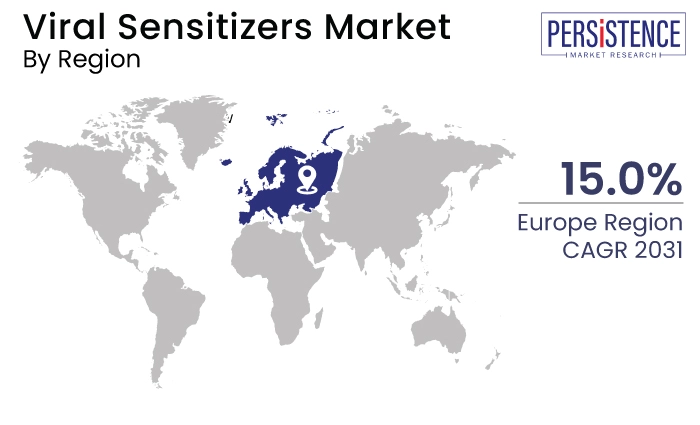
Europe |
15.0% |
Robust Research Foundation Accounts for North America’s Stronghold
North America is leading in the viral sensitizers market primarily due to its advanced healthcare infrastructure, robust research and development capabilities, and supportive regulatory environment.
The region boasts world-class healthcare facilities, research institutions, and pharmaceutical companies that drive innovation in biotechnology and therapeutic development.
Institutions in North America have spearheaded groundbreaking research in viral sensitizers, leveraging technologies like gene editing and synthetic biology to enhance the specificity and effectiveness of viral therapies.
Moreover, the clear and well-established regulatory pathways, such as those overseen by the FDA in the United States, facilitate efficient approval processes for new therapies, encouraging investment and accelerating market entry.
With high prevalence of chronic diseases and substantial healthcare expenditures, North America presents a fertile ground for the adoption of advanced therapies like viral sensitizers, positioning the region at the forefront of global market leadership.
Europe Gains from Well Developed Healthcare Infrastructure
Europe is emerging as a significant player in the market due to several key factors. The region benefits from a strong biomedical research infrastructure, encompassing leading academic institutions, biotechnology clusters, and healthcare centers that foster innovation.
European countries prioritize healthcare innovation and research funding, supporting advancements in biotechnological tools and genomic technologies essential for developing viral sensitizer therapies.
Additionally, the regulatory environment in Europe, governed by agencies like the European Medicines Agency (EMA), provides a clear framework for clinical trials and product approvals, ensuring rigorous yet predictable pathways to market entry.
Collaborations, and partnership to develop innovative products and accelerate the grant by regulatory bodies are the key growth strategies followed by the key players in the market. Companies are continuously investing in research and development to introduce innovative and break-through viral sensitizers products.
January 2023
FUJIFILM Irvine Scientific, Inc., a world leader in the development and manufacture of serum-free and chemically defined cell culture media for bioproduction and cell therapy manufacturing, announced the launch of its BalanCD HEK293 Viral Feed. The chemically defined, nutrient-based HEK293-specific feed medium is designed to boost adeno-associated viral vector (AAV) production for gene therapy applications and viral vector-based vaccines.
November 2023
Takara Bio Inc., an innovative biotechnology company, announced the developed of a novel adeno-associated virus (AAV) vector, SonuAAV™, which exhibits high gene transfer efficiency into inner ear tissues, in collaboration with Dr. Kazusaku Kamiya, Associate Professor at the Department of Otorhinolaryngology in Juntendo University School of Medicine.
|
Attributes |
Details |
|
Forecast Period |
2024 to 2031 |
|
Historical Data Available for |
2018 to 2023 |
|
Market Analysis |
US$ Billion for Value |
|
Key Regions Covered |
|
|
Key Market Segments Covered |
|
|
Key Companies Profiled in the Report |
|
|
Report Coverage |
|
|
Customization & Pricing |
Available upon request |
By Application
By End User
By Region
To know more about delivery timeline for this report Contact Sales

Thermo Fisher Scientific, FUJIFILM Irvine Scientific, Virica Biotech Tocris Bioscience, Takara Bio Inc., etc.
Oncolytic Viral Therapies segment records a significant market share as of 2024 in Application category.
Viral sensitizers are increasingly recognized for their potential in enhancing the effectiveness of oncolytic viruses and other cancer therapies.
The pharmaceutical companies segment records a significant market share as of 2024.