Comprehensive Snapshot of Therapeutic Drug Monitoring Market Research Report, Including Regional and Country Analysis in Brief.
Industry: Healthcare
Published Date: April-2025
Format: PPT*, PDF, EXCEL
Delivery Timelines: Contact Sales
Number of Pages: 190
Report ID: PMRREP35238
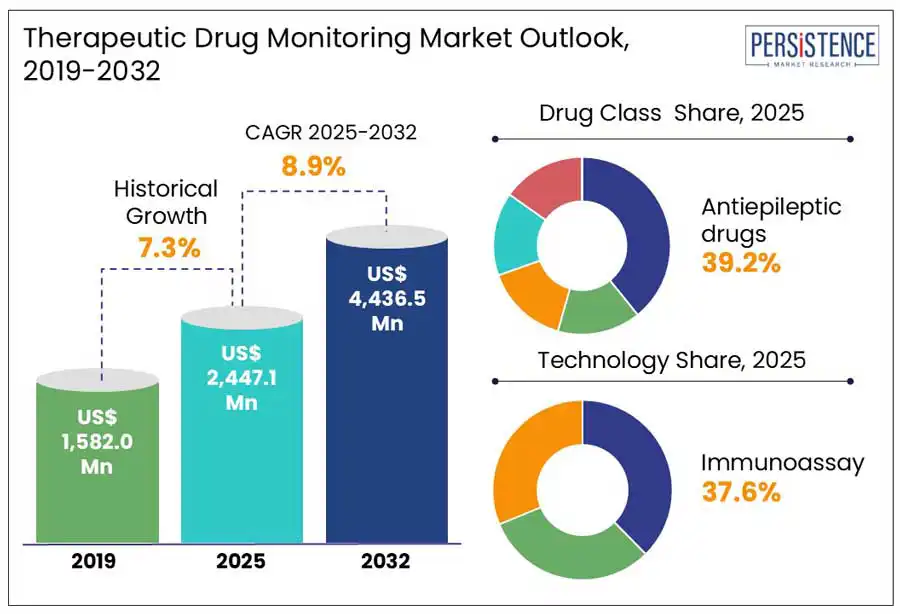
The global therapeutic drug monitoring market size is anticipated to rise from US$ 2.4 Bn in 2025 to US$ 4.4 Bn at a CAGR of 8.9% by 2032. According to the Persistence Market Research report, the increasing prevalence of chronic diseases coupled with growing demand for personalized treatment plans necessitates optimal drug levels and minimizing adverse effects.
Key Industry Highlights:
|
Global Market Attribute |
Key Insights |
|
Therapeutic Drug Monitoring Market Size (2025E) |
US$ 2.4 Bn |
|
Market Value Forecast (2032F) |
US$ 4.4 Bn |
|
Projected Growth (CAGR 2025 to 2032) |
8.9% |
|
Historical Market Growth (CAGR 2019 to 2024) |
7.3% |
Organ transplant recipients need to take immunosuppressant medications for their entire lives to avoid rejection of the transplanted organ. Therapeutic drug monitoring (TDM) is essential to maintain appropriate medication levels which helps minimize the chance of toxicity and failure of the transplant. The rising global incidence of organ transplants is leading to a growing need for TDM in the field of transplant medicine. In 2024, Siemens Healthineers broadened its therapeutic drug monitoring (TDM) offerings to feature an extensive array of immunosuppressant drug assays. These assays aim to provide precise measurement of drug levels in patients receiving immunosuppressive treatment, thus enhancing treatment effectiveness and reducing toxicity.
The adoption of therapeutic drug monitoring (TDM) faces considerable logistical obstacles, mainly due to the high expenses related to advanced analytical equipment, specific consumables, and the necessity for trained staff. TDM necessitates advanced instruments like high-performance liquid chromatography (HPLC) and mass spectrometry, which comes with significant acquisition and maintenance costs.
Furthermore, laboratories are required to comply with rigorous quality control regulations, which adds to the operational expenses. These challenges are especially severe in semi-urban and rural regions where healthcare facilities are underdeveloped. The scarcity of qualified personnel and financial limitations further hinder the broad implementation of TDM services.
Therapeutic Drug Monitoring (TDM) in oncology offers a significant opportunity as personalized chemotherapy dosing through TDM helps optimize drug efficacy by minimizing toxicity, especially for narrow therapeutic windows like methotrexate and platinum-based drugs. With rising cancer incidence and precision medicine adoption, demand for TDM is expected to grow In June 2024, Baysient launched TuMinimize, a web-based nomogram intended to enhance the dosing of oncology biologics, illustrating the industry’s commitment to advancing personalized oncology care. This precision dosing enhances patient outcomes and reduces hospitalization costs due to toxicity-related complications.
By drug class, the antiepileptic drugs segment is expected to dominate with a projected share of 39.2% in 2025. This is primarily because these medications have a narrow therapeutic index, requiring precise dosage adjustments to maximize effectiveness while reducing toxicity. Continuous monitoring is vital for individuals with epilepsy, thereby increasing the need for TDM solutions. In 2023, Chromsystems introduced the "Parameter Set Antiepileptic Drugs All-in-One Method - LC-MS/MS," a complete kit made to identify different antiepileptic medications in serum and plasma samples.
By technology, the immunoassays segment is likely to hold a share of 37.6% in 2025. Immunoassays hold the leading position in the TDM market because of their excellent sensitivity, specificity, and quick turnaround time, which makes them well-suited for the regular monitoring of AEDs, immunosuppressants, and antibiotics. Additionally, their automation, cost-efficiency, and alignment with hospital workflows further enhance their usability. In April 2024, Beckman Coulter unveiled the DxI 9000 Immunoassay Analyzer, which is intended to improve diagnostic testing speed and accuracy, including therapeutic drug monitoring (TDM) applications. By delivering accurate and rapid data, this advanced analyzer seeks to enhance patient care and promote efficient TDM procedures.
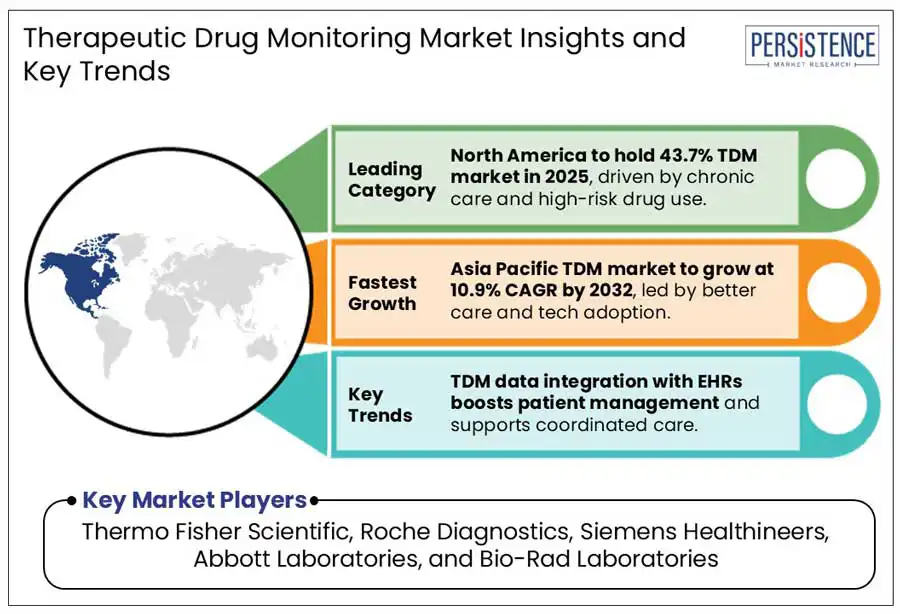
North America therapeutic drug monitoring market is projected to register a share of 43.7% in 2025. TDM is commonly utilized in fields such as transplant medicine, oncology, infectious diseases, neurology, and cardiology to enhance drug treatment and avoid adverse effects. It ensures optimal dosing for immunosuppressive agents, chemotherapy drugs, antibiotics, antiepileptic medications, and anticoagulants, preventing toxicity and improving efficacy. In 2023, Ferring introduced Rebyota and Adstiladrin in the United States, expanding into different therapeutic domains. These new developments could impact the therapeutic drug monitoring market by presenting innovative treatment strategies that necessitate specialized monitoring.
Europe therapeutic drug monitoring market is expected to account for 29.1% share by 2025. TDM is being used more frequently to customize drug dosages, especially for critical medications such as antibiotics, chemotherapy agents, and immunosuppressants. Regulatory bodies and research institutions in Europe are supporting the integration of AI-driven analytics and real-time monitoring technologies, enhancing accuracy and efficacy. The European Medicines Agency (EMA) is essential in overseeing medications in the EU, guaranteeing that evaluation, supervision, and safety monitoring meet high standards. The regulatory framework established by the EMA encourages the incorporation of innovative therapeutic drug monitoring (TDM) practices into clinical environments, aiding the shift from innovation to practical application within European healthcare systems.
Asia Pacific therapeutic drug monitoring market is estimated to account for a share of 19.6% in 2025. Emerging availability of healthcare services and enhanced patient care are propelling the growth of therapeutic drug monitoring (TDM) market in the Asia-Pacific area. Developing healthcare infrastructure, rising uptake of precision medicine, and escalating awareness among healthcare professionals regarding personalized drug therapy are significant elements boosting TDM execution. In 2018, According to NIH, A survey was conducted of 2 hospitals in China, hospital A recorded 3.53 million outpatient visits along with 17,820 TDM cases, whereas hospital B reported 3.5 million visits with 6,916 cases. The low submission rate reflects a restricted clinical uptake of TDM. National efforts, such as the EQA Investigation Plan for 33 drugs.
Furthermore, progress in laboratory technologies, regulatory support, and the arrival of novel monitoring techniques such as dried blood spot testing and molecular diagnostics are enhancing the precision, accessibility, and effectiveness of TDM throughout the region.
The global therapeutic drug monitoring market is fiercely competitive, with major players such as Thermo Fisher Scientific, Roche Diagnostics, Siemens Healthineers, Abbott Laboratories, and Bio-Rad Laboratories emphasizing innovation, collaborations, and product growth. Forming strategic partnerships with pharmaceutical companies is vital for establishing enduring alliances in drug monitoring solutions. Regulatory hurdles and adherence to FDA, EMA, and ISO standards significantly influence market entry and development.
North America and Europe lead due to well established healthcare systems, while Asia Pacific is emerging as a rapidly expanding market, fueled by the rising investments in healthcare and heightened awareness.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis Units |
Value: US$ Bn, Volume: Units |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Product
By Sample Type
By Drug Class
By Technology
By End-use
By Region
To know more about delivery timeline for this report Contact Sales

The market is set to reach US$ 2.4 Bn in 2025.
Increasing importance of therapeutic drug monitoring in immunosuppressive treatment following transplantation is the key demand driver for the therapeutic drug monitoring market
The industry is estimated to rise at a CAGR of 8.9% through 2032.
North America is projected to hold the largest industry share in 2025.
Thermo Fisher Scientific, Roche Diagnostics, Siemens Healthineers, Abbott Laboratories, and Bio-Rad Laboratories Incorporated are a few leading players.