RNA-based Therapeutics and Vaccines Market Segmented By RNA-based Therapeutics and RNA-based Vaccines with Oncology, Immunology, Ophthalmology, Cardiovascular Diseases, Infectious Diseases, Genetic Diseases Indication
Industry: Healthcare
Published Date: May-2022
Format: PPT*, PDF, EXCEL
Delivery Timelines: Contact Sales
Number of Pages: 177
Report ID: PMRREP12868
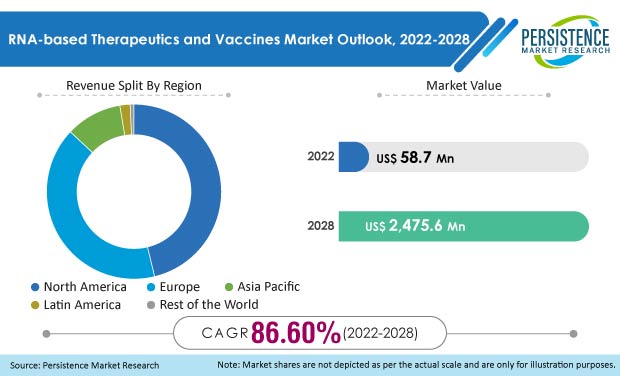
The global RNA-based therapeutics and vaccines market had witnessed a staggering CAGR of 51.6% between 2013 and 2021. As of the year 2022, it is expected to reach US$ 58.7 Mn and US$ 2.48 Bn by the year 2028, thereby recording a whopping 86.6% CAGR between 2022 and 2028.
| Attribute | Key Insights |
|---|---|
|
RNA-based Therapeutics and Vaccines Market (2022) |
US$ 58.7 Mn |
|
Projected Market Value (2028) |
US$ 2.48 Bn |
|
Global Market Growth Rate (2022-2028) |
86.6% CAGR |
|
Market Share of North America and Europe |
87% |
The global RNA-based Therapeutics and Vaccines Market was valued at US$ 8.99 Mn in the year 2019. It is expected to grow almost six-fold by the year 2022.
RNA therapeutics consist of 4 types – RNA aptamers, RNA interference (RNAi), antisense RNA (asRNA), and messenger RNA (mRNA). Out of these, therapy based on mRNA is the only one wherein protein synthesis gets triggered within the cells. This renders it useful in development of vaccine.
RNA-based therapeutics and vaccines started tasting success during early 2010s. The years 2020 and 2021 saw development of mRNA vaccines for mitigating Covid-19 pandemic. The very first mRNA vaccine duly approved by medicines regulator was Pfizer-BioNTech Covid-19 vaccine. Moderna Covid-19 vaccine followed suit.
It is a known fact that the features looked upon with regards to ideal delivery of drugs include biocompatibility, controlled distribution (location and persistence), guarding against nucleases, and safety.
As of the year 2022, the Asia-Pacific accounts for 10.4% of the RNA-based Therapeutics and Vaccines market. This percentage is bound to increase in the forecast period with increasing awareness amongst the people and extensive funding from the developing economies herein.
“Oncology to Be the Focal Point of RNA-Based Therapeutics and Vaccines Market”
RNA-based therapeutics and vaccines, better known as RNA-based biopharmaceuticals, come across as a refined type of treatment and prophylactic for numerous rare and chronic ailments inclusive of cancer, tuberculosis, diabetes, and cardiovascular diseases.
Research states that out of nearly 700 RNA and DNA therapeutics in pipeline, around 430 are in initial stages of development. On the top of that, close to 35% are emphasizing on oncology. At the global level, there are approximately 65 universities and 160 organizations into development of therapies based on RNA.
North America and Europe (combined) are expected to be worth US$ 51.07 Mn in the year 2022.
“Manufacturers to Ride on Stability Provided by RNA Therapeutics and Vaccines”
RNA therapeutics and vaccines hold the distinction of providing better stability as compared to their counterparts. Though majority of them are under trials (as mentioned above), they are being increasingly preferred when it comes to treat obstinate disorders. The technologies that are being readily adopted include Smart technology, antisense technology, and RNAi interference technology. RNAi and antisense technology have been drawing attention of the researchers as they help in provision of base sequence for producing RNA drugs. However, it should not be inferred that both the technologies have the same way of functioning. Antisense technology works by creation of RNA strand from a known gene sequence, whereas RNAi technology implies destruction of specific mRNA molecules.
“Expensive Research to be the Major Restraint”
RNA-based therapeutics and vaccines market is subject to costly research, followed by higher rate of failure during clinical trials. Also, issues regarding drug delivery can’t be ruled out. Higher costs involved in research could compel the under-developed economies to give a second thought regarding making investments into this market.
“The US to Dominate the RNA-based Therapeutics and Vaccines Market”
The US is on the top pedestal as far as RNA-based therapeutics and vaccines market is concerned. This could be reasoned with quick adaptation to technologies coupled with growing incidences of chronic diseases. Plus, a handsome investment is being done in research by the government regarding advancements in the RNA-based technologies.
Coming to Europe, Germany, the UK, and Belgium rule the market due to continuous advancements on the technological front.
The Asia-Pacific is expected to hold a significant market share in the forecast period due to the market players drawing realization that overheads come out to be less.
“The Market categorized based on Type of Product, Application, and End-user”
The RNA-based Therapeutics and Vaccines Market, by application, spans research applications, diagnostic testing, and clinical testing. By end-user, it’s diagnostic centers, hospitals, academic & research institutes, forensic science laboratories, and CROs (Contract Research Organizations). By type of product, it’s workstations, reagents and consumables, and kits (RNA sample preparation and DNA sample preparation).
“Clinical Testing, Academic & Research Institutes, and Kits leading from the Front”
These days, clinical testing is the need of the hour, as various chronic ailments, and most importantly, the pandemic needs to be brought under control. The academic & research institutes are engaged in their entirety in lending a constructively helping hand in RNA-based therapeutics and vaccines. Kits are preferred as the holistic package serves a greater purpose, including the history and the then course of action. However, they should be kept away from contamination, or else the analysis won’t be precise.
“Covid-19 having left No Stone Unturned in Keeping the Market on the Toes”
The healthcare vertical has witnessed maximum turmoils during the pandemic, and they do not seem to stop any time soon. Research is being conducted in almost every part of the developed world regarding the ways in which Covid-19 could be brought under control. Now that the world is grappling with the third wave in the form of Omicron variant, there is no relaxation for RNA-based therapeutics and vaccines. The only major difference is that with chronic diseases, Covid-19 has also figured in the list of “mandatory combating of diseases”.
The key players are going for every possible mode of expansion, inclusive of mergers, acquisitions, new therapeutics’ and vaccines’ launch, joint ventures, partnerships,, and likewise for making a mark in the market.
One such development is as follows –
| Attribute | Details |
|---|---|
|
Forecast Period |
2022-2028 |
|
Historical Data Available for |
2013-2021 |
|
Market Analysis |
US$ Mn/Bn for Value |
|
Key Regions Covered |
|
|
Key Countries Covered |
|
|
Key Market Segments Covered |
|
|
Key Companies Profiled |
|
|
Pricing |
Available upon Request |
RNA-based Therapeutics and Vaccines Market by Product Type
RNA-based Therapeutics and Vaccines Market by Indication Type
RNA-based Therapeutics and Vaccines Market by Region
To know more about delivery timeline for this report Contact Sales

The RNA-based Therapeutics and Vaccines Market is expected to be worth US$ 58.7 Mn in the year 2022 and grow 42.1 times by the year 2028.
The market for RNA-based Therapeutics and Vaccines is expected to reach US$ 2.48 Bn by 2028, expanding at a CAGR of 86.6%.
North America is one of the highest revenue generators for RNA-based Therapeutics and Vaccines, accounting for 46.2% of the global market share.
Alnylam Pharmaceuticals, Inc., Arbutus Biopharma Corp., Arrowhead Pharmaceuticals, Inc., BioNTech AG, CureVac AG, Dicerna Pharmaceuticals, Inc., Regulus Therapeutics, Inc., Marina Biotech, Inc., miRagen Therapeutics, Moderna Therapeutics, Inc., Quark Pharmaceuticals, Inc., Santaris Pharma A/S (A Roche Company), and Sylentis S.A.
The Asia-Pacific’s RNA-based Therapeutics and Vaccines market holds 10.4% of the market share.