Comprehensive Snapshot of Refurbished Medical Equipment Research Report, Including Regional and Country Analysis in Brief.
Industry: Healthcare
Published Date: April-2025
Format: PPT*, PDF, EXCEL
Delivery Timelines: Contact Sales
Number of Pages: 170
Report ID: PMRREP35242
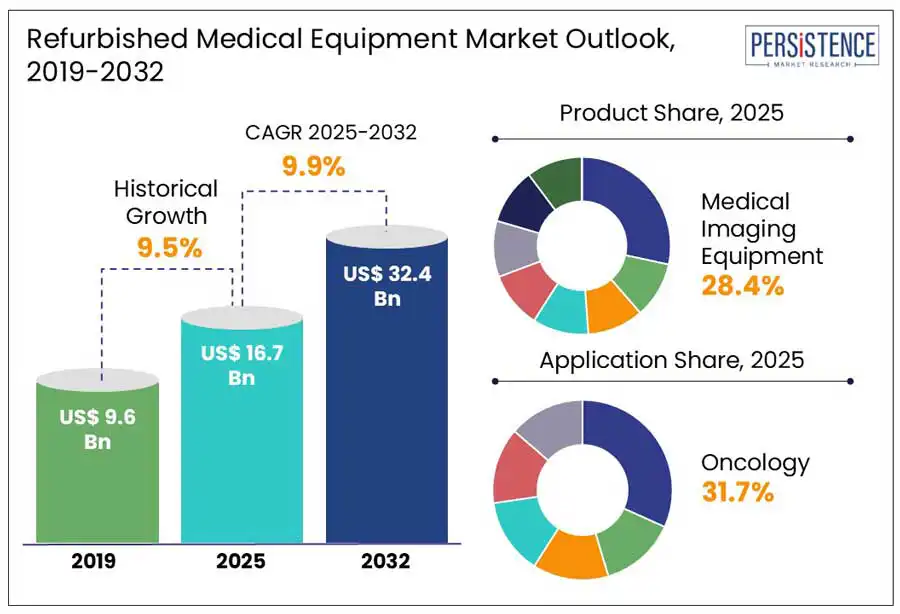
The global refurbished medical equipment market size is anticipated to rise from US$ 16.7 Bn in 2025 to US$ 32.4 Bn by 2032. It is projected to witness a CAGR of 9.9% from 2025 to 2032.
As per Persistence Market Research (PMR), refurbished medical devices are a rapidly growing segment within the global healthcare industry, driven by increasing demand for cost-effective solutions without compromising on quality. According to World Health Organization (WHO), in 2022, global health expenditures reached $9.8 trillion, equivalent to 9.9% of the world's GDP. Medical goods accounted for 14%–15% of total health spending in both high-income and middle-income countries.
Key Industry Highlights:
|
Global Market Attribute |
Key Insights |
|
Refurbished Medical Equipment Market Size (2025E) |
US$ 16.7 Bn |
|
Market Value Forecast (2032F) |
US$ 32.4 Bn |
|
Projected Growth (CAGR 2025 to 2032) |
9.9% |
|
Historical Market Growth (CAGR 2019 to 2024) |
9.5% |
The growing concerns over medical e-waste and the increasing emphasis on sustainable healthcare practices are key drivers of the refurbished medical device market. According to WHO, in 2022, global e-waste production reached an estimated 62 million tons, with only 22.3% formally collected and recycled.
Hospitals generate significant amounts of discarded medical equipment, contributing to pollution and resource depletion. To combat this, according to Practice Greenhealth, in 2022, 68% of hospitals considered a sustainable procurement policy when making purchasing decisions, and 53% set sustainable procurement goals for their organizations.
Refurbishing extends device lifespans and reduces waste. With sustainability gaining importance, healthcare providers are choosing refurbished devices as a cost-effective and eco-friendly alternative to new equipment.
The U.S. Food and Drug Administration (FDA), in 2024, expressed concerns about the quality of servicing for medical devices, particularly refurbished ones. Poor servicing results in device malfunctions and poses risks to patient safety. To address this, the FDA issued guidance clarifying the definition of "remanufacturing" for reusable devices needing maintenance or repair.
This guidance ensures consistency in remanufacturing practices and reinforces that entities involved in remanufacturing are considered manufacturers. They must comply with regulatory requirements, including registration, listing, adverse event reporting, and adherence to the Quality System regulation, all aimed at maintaining the safety and effectiveness of refurbished medical devices.
The refurbished medical equipment market is growing rapidly as private healthcare institutions seek cost-effective solutions. Budget constraints and rising demand for advanced devices have made refurbished options more appealing. Furthermore, the growth of medical tourism and expanding private healthcare infrastructure in emerging economies drive this demand.
In February 2025, Spire Healthcare, a leading private hospital group in the UK, invested in refurbished facilities and equipment to enhance service delivery. Their newly refurbished hospital near Gatwick features a £1.5 million CT scanner, underscoring the private sector's role in adopting refurbished medical devices to meet growing healthcare demands. As more private institutions prefer refurbished equipment, market players have significant opportunities for growth and expansion.
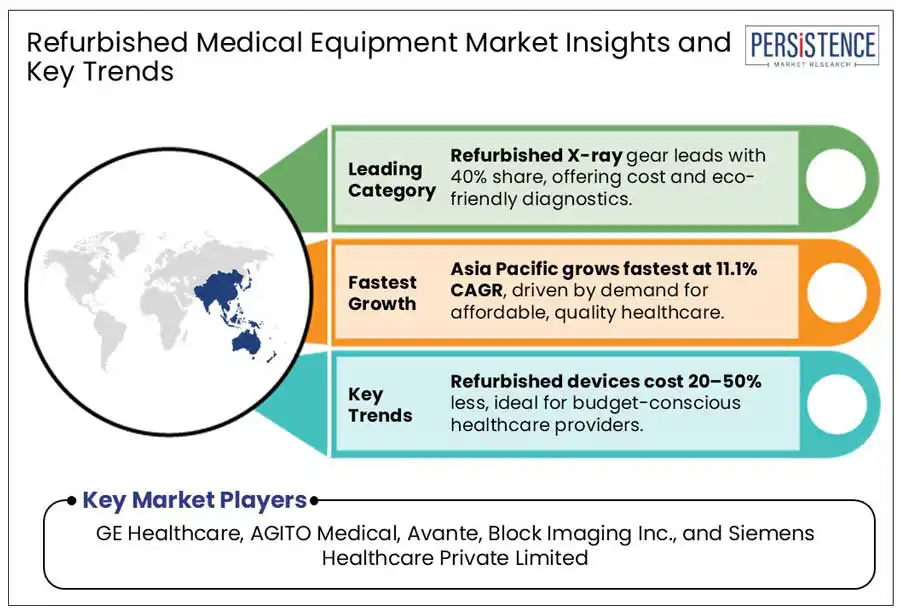
Refurbished X-ray equipment is a cost-effective solution for healthcare organizations, providing access to critical diagnostic instruments while also supporting environmental sustainability. Refurbished equipment generally costs between 30% and 70% less than new equipment, making it an excellent option for facilities with limited budgets. This cost savings allows healthcare providers to allocate funds to other critical areas, including patient care,staffing, and additional technology. However, tight laws govern the use and distribution of such technology to assure its safety and usefulness. In India, the Atomic Energy Regulatory Board (AERB) has particular requirements for importing and using pre-owned medical diagnostic X-ray equipment. According to AERB guidelines, pre-owned X-ray equipment older than seven years must be discontinued for exports.
With growing oncology cases worldwide, demand for affordable and high-quality refurbished medical devices in cancer care continues to rise. According to the Pan American Health Organization (PAHO) globally in 2023, there were an estimated 20 million new cases of cancer and 10 million deaths from cancer. The cancer burden is estimated to increase by approximately 60% over the next two decades, further straining health systems.
The oncology segment drives the refurbished medical equipment market due to the increasing global cancer burden and the demand for affordable, quality treatments. Refurbished radiotherapy equipment, such as linear accelerators and brachytherapy systems, enables healthcare providers to offer advanced treatments at reduced costs. According to WHO, more than 50% of cancer patients require radiotherapy as part of cancer care, and it is frequently used to treat the most common types, such as breast, cervical, colorectal, and lung cancer.
North America refurbished medical equipment market is projected to hold a share of 38.1% in 2025. As healthcare costs rise, small and mid-sized hospitals choose affordable alternatives over expensive devices. Refurbished imaging systems, surgical tools, and patient monitors help maintain quality care while optimizing budgets. Strict FDA and third-party standards ensure these devices meet safety and performance criterion. For instance, in August 2024, the U.S. FDA implemented stringent regulations to ensure the safety and effectiveness of reprocessed medical devices, including those labeled for single use.
The Europe refurbished medical equipment market is witnessing significant growth due to the rising geriatric population and increasing demand for low-cost medical devices. With a growing number of elderly individuals requiring continuous healthcare services, hospitals and diagnostic centers are seeking cost-effective solutions to meet demand while managing healthcare expenditures. According to Eurostat’s January 2023 statistics, the European Union (EU) population was estimated at 448.8 million people and more than one-fifth (21.3 %) of it was aged 65 years and over.
The EU and national agencies are also promoting sustainable procurement policies that support the reuse of medical devices under strict refurbishment regulations. With healthcare costs escalating and budget constraints in public hospitals, the adoption of certified refurbished medical equipment is expanding.
Low-income countries in the Asia Pacific are turning to refurbished medical equipment to improve healthcare access while staying on budget. With limited funding and high costs for new devices, nations like Bangladesh, Nepal, Cambodia, and Myanmar find refurbished options more viable, focusing on affordable imaging and surgical equipment, especially X-rays, ultrasounds, and patient monitoring systems.
In July 2024, WHO launched MeDevIS, an online platform providing comprehensive information on over 2,300 types of medical devices. This resource aids governments and healthcare providers, especially in resource-limited settings, in making informed decisions about the selection and procurement of medical technologies, potentially including refurbished equipment.
Government initiatives and NGO support are promoting refurbished medical technology to improve healthcare infrastructure and increase access to medical services in resource-limited areas.
The global refurbished medical equipment market is highly competitive, with key players focusing on technological upgrades, strategic partnerships, and regulatory compliance to enhance their market presence. Leading companies in this sector include GE Healthcare, Siemens Healthineers, Philips Healthcare, and Canon Medical Systems. These companies offer certified refurbished devices with warranties and quality assurance.
Smaller players and independent refurbishers compete by providing affordable solutions to hospitals and diagnostic centers, especially in cost-sensitive markets. The growing preference for eco-friendly healthcare solutions has further intensified competition. Additionally, regulatory bodies such as the FDA in the U.S. and MDR in the EU are enforcing strict quality standards, which are shaping the competitive landscape of the industry.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis Units |
Value: US$ Bn, Volume: Units |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Product
By Application
By End-user
By Region
To know more about delivery timeline for this report Contact Sales

The market is set to reach US$ 16.7 Bn in 2025.
Medical Imaging Equipment is considered a best-selling product.
The industry is estimated to rise at a CAGR of 9.9% through 2032.
North America is projected to hold the largest share of the industry in 2025.
GE HealthCare, AGITO Medical, and Avante are a few leading players.