Industry: Healthcare
Published Date: March-2025
Format: PPT*, PDF, EXCEL
Delivery Timelines: Contact Sales
Number of Pages: 173
Report ID: PMRREP25768
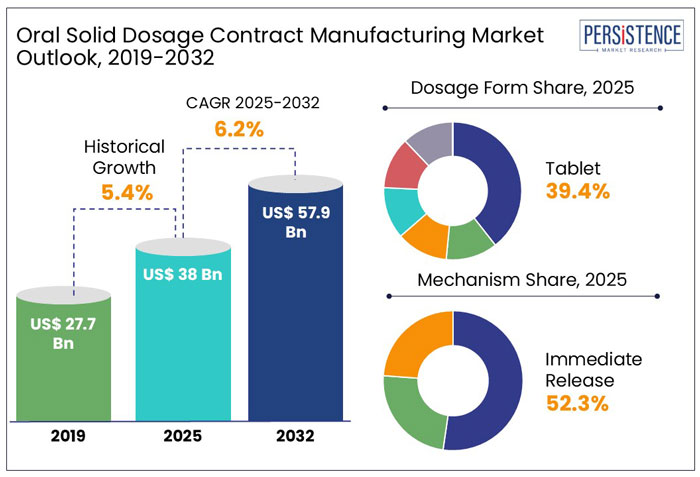
The global oral solid dosage contract manufacturing market size is anticipated to rise from US$ 38 Bn in 2025 to US$ 57.9 Bn by 2032. It is projected to witness a CAGR of 6.2% from 2025 to 2032.
Advancements in drug delivery technology, including targeted dosage forms and 3D printing, are transforming the oral pharmaceuticals market, improving patient outcomes and ensuring precise dosage control.
The production of solid dosage pharmaceuticals is growing because it is efficient and economical; digital technologies improve process monitoring and quality control, while controlled-release technologies increase bioavailability.
With the goal of improving patient adherence, CDMOs (Contract Development and Manufacturing Organizations) are advancing the development of next-generation oral medications using advanced manufacturing technologies.
Key Highlights of the Oral Solid Dosage Contract Manufacturing Market
|
Global Market Attributes |
Key Insights |
|
Oral Solid Dosage Contract Manufacturing Market Size (2025E) |
US$ 38 Bn |
|
Market Value Forecast (2032F) |
US$ 57.9 Bn |
|
Projected Growth (CAGR 2025 to 2032) |
6.2% |
|
Historical Market Growth (CAGR 2019 to 2024) |
5.4% |
Collaborative Efforts of Healthcare Sector Caters Innovation in Drug Development
The global oral solid dosage contract manufacturing industry witnessed a CAGR of 5.4% in the historical period between 2019 and 2024, as per Persistence Market Research. The need for novel treatments has increased because of the rising incidence of chronic and infectious illnesses. Oral solid dose contract manufacturing is increasing due to major pharmaceutical corporations outsourcing contract production.
In order to increase their market presence and take advantage of common technologies, businesses are concentrating on acquisitions and mergers. For instance,
Scientific Advances in Oral Solid Dosage Forms to Boost Sales through 2032
In the estimated timeframe from 2025 to 2032, the global market for oral solid dosage contract manufacturing is likely to showcase a CAGR of 6.2%. There are a lot of prospects for oral solid dosage contract manufacturing organizations throughout the world over the projection period. These businesses can concentrate on developing new technologies to enhance the morphological properties, stability, yield, and bioavailability of oral solid dosage forms.
Market players can create a variety of oral solid dosage forms, including controlled release, extended-release, and enteric-coated. For instance, using its GPEx Boost platform, Catalent's Biologics domain provides cell-line development services to produce stable, high-yielding mammalian cell lines for biosimilars.
However, because of the market's fragmentation and plenty of small CMOs, consolidation presents a substantial chance for quicker development. Big and small pharmaceutical and biotech companies are drawn to flexible business models developed by contract development and manufacturing companies. Particle engineering is a tactic that CDMOs can use to increase their market share.
Growth Drivers
Enhanced Scalability and Reduced Costs Fueling Demand for OSD CMO
Contract Manufacturing Organizations (CMOs) that specialize in oral solid dosage are becoming increasingly in demand because of their improved scalability, cost-effectiveness, and innovations in medication manufacture, which aid pharmaceutical businesses in streamlining their operations. For instance,
The pharmaceutical industry is predicted to grow significantly worldwide, necessitating the smart outsourcing of OSD manufacturing. Continuous production, automation, and AI-driven quality control are all combined to increase productivity and lower costs, which makes OSD contract manufacturing a fast-growing market.
Risk of Non-Compliance and Quality Issues as Barriers in OSD Contract Manufacturing
The OSD Contract Manufacturing industry is anticipated to have issues with quality control and regulatory compliance, which might impede market expansion. Production halts, product recalls, and financial penalties can result from strict GMP regulations enforced by organizations such as the FDA, EMA, and PMDA.
Small contract development and manufacturing organizations often struggle with high regulatory costs, complex documentation, and stringent inspections, resulting in longer drug approval timelines and slowed market expansion in developed nations. For instance,
To mitigate risks, CDMOs are forming strategic partnerships and expanding globally, like Lonza's Singapore expansion in October 2021. However, outsourcing to low-cost markets like India and China presents challenges like supply chain disruptions, regulatory differences, and communication barriers.
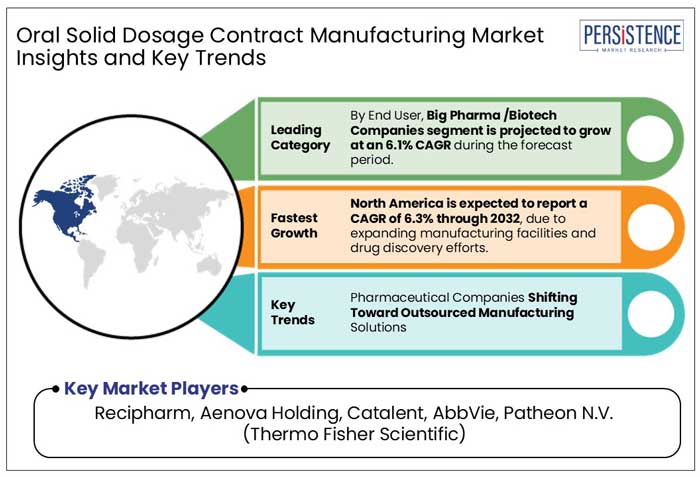
Pharmaceutical Companies Shifts Toward Outsourced Manufacturing Solutions
Pharmaceutical companies are outsourcing manufacturing to CDMOs to improve efficiency, reduce costs, and meet demand for specialized drug formulations due to the complexity of biologics, specialty drugs, and oral solid dosage forms. CDMOs offer advanced technology, regulatory expertise, and large-scale production capabilities. For example,
In order to concentrate on their core skills, pharmaceutical firms are outsourcing their manufacturing processes. The biopharma outsourcing industry is expected to expand quickly, and CDMOs are essential to the development of new medicinal products.
Dosage Form Insights
Tablets Gain Popularity with Ease of Administration in Healthcare
Tablets are the dominant form in the oral solid dosage contract manufacturing market, accounting for 39.4% of the market share in 2025. They are preferred due to cost-effectiveness, ease of administration, and controlled drug release. Leading pharmaceutical CDMOs, including Catalent, Lonza, and Recipharm, continue to invest in tablet production to meet growing demand. For instance,
Additionally, regulatory agencies such as the FDA and EMA are pushing for enhanced manufacturing processes, driving innovation in coating technologies, bioavailability enhancement, and excipient optimization. With the demand for oral solid dosage drugs across chronic disease management, oncology, and infectious diseases, the tablet segment is expected to maintain its dominant position in the global OSD contract manufacturing market.
End User Insights
Big Pharma and Biotech Companies Pushes OSD Contract Manufacturing Growth
In 2025, big pharma and biotech companies are expected to account for 43.4% of the end-user market share in OSD contract manufacturing. These companies increasingly rely on CDMOs to scale production, access specialized expertise, and accelerate drug development. The segment is projected to report a CAGR of 6.1% from 2025 to 2032, driven by rising investments in research and development and outsourcing trends.
To meet this demand, Lonza announced in March 2024 the expansion of its OSD manufacturing capacity in Switzerland, focusing on high-potency APIs and customized solid dose formulations.
Similarly, Catalent invested US$ 175 Mn in February 2024 to enhance its U.S. facilities for large-scale tablet and capsule production, supporting biotech firms with advanced formulation solutions.
Regulatory pressures and the high costs of in-house manufacturing push Pfizer, Novartis, and AstraZeneca to partner with CDMOs, ensuring compliance while optimizing costs. The growth of biologics and targeted therapies further solidifies CDMOs’ role in delivering scalable, cost-efficient OSD manufacturing.
North America Presents Prospects for Innovations in Pharmaceuticals Development
In 2025, North America is projected to account for 40.5% of the global market, driven by expanding pharmaceutical manufacturing facilities and increased drug discovery efforts. The market is forecasted to report a CAGR of 6.3% from 2025 to 2032, supported by novel government initiatives and innovation in OSD formulations.
The region’s pharmaceutical sector continues to invest in advanced manufacturing capabilities, with CMOs playing a crucial role in ensuring scalable production and quality control. For instance,
The growing reliance of pharmaceutical companies on CMOs for expertise, cost efficiency, and rapid production is expected to drive North America’s market dominance in the global healthcare sector.
Outsourcing of Pharmaceutical Manufacturing Develops Opportunities in the Asia Pacific
In 2025, Asia Pacific is expected to become a significant region due to improved social insurance schemes and rising outsourcing projects in countries like China, India, and Singapore to strengthen pharmaceutical manufacturing.
In addition, Singapore has emerged as a hub for high-quality pharmaceutical manufacturing, with companies like Lonza and Pfizer investing in advanced OSD manufacturing.
Asia Pacific's cost-effective production, skilled workforce, and regulatory advancements attract international pharmaceutical firms, making it a powerhouse in contract manufacturing and drug development.
Europe Cultivates Innovative Research in Pharmaceutical Drug Manufacturing
Europe is set to boost pharmaceutical drug manufacturing research and development through increased investments and strategic acquisitions, leveraging its position as a hub for innovation.
Europe's regulatory agencies, such as the EMA, promote innovation by enticing pharmaceutical businesses to invest in new technologies, presenting the region as a leader in next-generation manufacturing, increasing drug development efficiency, and boosting its worldwide healthcare role.
The global oral solid dosage contract manufacturing market is fiercely competitive, with leading companies dominating the landscape through their integrated business operations. Key companies are set to focus on new product launches with unique characteristics, strengthening distribution networks, as well as geographical expansions.
The increasing demand for pharmaceutical manufacturing is driving companies to expand their manufacturing units, while key providers of oral solid dosage contract manufacturing products are utilizing acquisitions and expansion strategies to strengthen their market presence in the forthcoming period.
Key Industry Developments
|
Report Attributes |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis Units |
Value: US$ Bn/Mn, Volume: As applicable |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Dosage Form
By Mechanism
By Application
By End User
By Region
To know more about delivery timeline for this report Contact Sales

The market is set to reach US$ 38 Bn in 2025.
The trends in oral solid dosage contract manufacturing include continuous manufacturing, 3D-printed personalized medicine, and high-potency APIs.
Recipharm, Aenova Holding, Catalent, AbbVie, Patheon N.V. (Thermo Fisher Scientific) are a few leading players.
The industry is estimated to rise at a CAGR of 6.2% through 2032.
North America is projected to hold the largest share of the industry in 2025.