ID: PMRREP16789| 195 Pages | 5 Mar 2025 | Format: PDF, Excel, PPT* | Healthcare

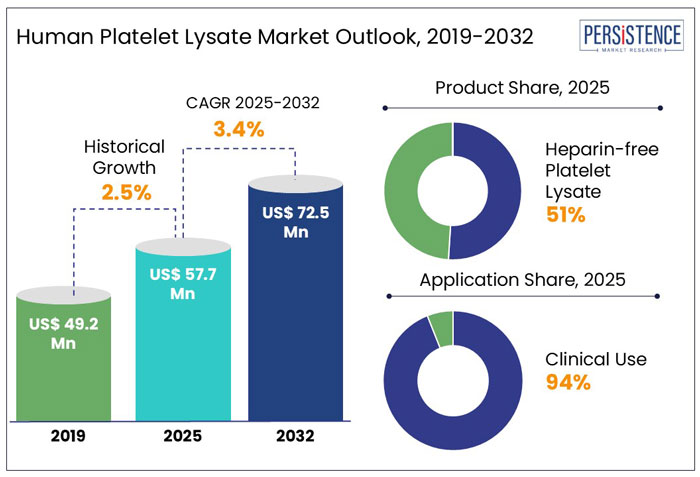
The global human platelet lysate market size is anticipated to rise from US$ 57.7 Mn in 2025 to US$ 72.5 Mn by 2032. It is projected to witness a CAGR of 3.4% from 2025 to 2032.
In the evolving landscape of regenerative medicine, Human Platelet Lysate (HPL) has emerged as a pivotal player, transforming cell culture and therapeutic applications.
Consider a biotech startup aiming to develop therapies with minimal risk of adverse reactions. They turn to heparin-free HPL, which has become the preferred choice for many in the industry.
In 2022, this segment held a market share of approximately 51%, underscoring its prominence in both research and clinical settings.
The increasing incidence of chronic conditions has led to an increased demand for advanced therapeutic solutions, thereby boosting the adoption of HPL in various treatments. Continuous research and development in regenerative therapies have expanded the applications of HPL, further driving market growth.
Key Highlights of the Human Platelet Lysate Market
|
Global Market Attributes |
Key Insights |
|
Human Platelet Lysate Market Size (2025E) |
US$ 57.7 Mn |
|
Market Value Forecast (2032F) |
US$ 72.5 Mn |
|
Projected Growth (CAGR 2025 to 2032) |
3.4% |
|
Historical Market Growth (CAGR 2019 to 2024) |
2.5% |
Growing Need for Xeno-Free Alternatives Catered to Market Growth
The human platelet lysate (HPL) market steadily rose from 2019 to 2023, driven by the growing need for xeno-free alternatives in regenerative medicine.
Researchers sought reliable substitutes for fetal bovine serum (FBS), and HPL emerged as a game-changer, offering superior cell growth capabilities and enhanced safety. However, high production costs, supply chain limitations, and regulatory hurdles kept the market from reaching its full potential.
Despite these barriers, the industry saw an uptick in adoption, particularly in stem cell therapy and tissue engineering, paving the way for future growth.
Advancements in Bio-Manufacturing Propels Market Revenue
Over the forecast period, the market is transforming at an accelerated pace with the rapid advancements in bio-manufacturing, regulatory approvals, and increased healthcare investments.
Companies are developing scalable production methods, reducing costs, and ensuring consistent quality.
For instance, Sanofi announced an investment of over €1 billion in May 2024 to enhance bio-manufacturing capacities at its facilities in France, aiming to double monoclonal antibody production.
The global surge in regenerative medicine and cell therapy trials is further fueling demand. Government and private sector funding is expanding access to HPL, making it a staple in clinical and research applications.
Growth Driver
Rising Demand for Regenerative Medicine Augment Market Growth
Imagine a world where damaged tissues and organs can be repaired naturally without invasive surgeries. It is no longer a distant dream but a reality unfolding through regenerative medicine, a field that thrives on ground-breaking innovations like human platelet lysate (HPL).
As researchers and clinicians seek alternatives to traditional cell culture supplements, HPL is emerging as a game-changer. Unlike fetal bovine serum (FBS), which carries risks of immune reactions and ethical concerns, HPL provides a human-derived, xeno-free solution that enhances the growth and viability of stem cells.
The rising adoption of stem cell therapies for treating conditions like osteoarthritis, neurodegenerative diseases, and chronic wounds is fuelling the demand for HPL.
With governments and private investors pouring billions into regenerative medicine, the role of HPL is set to expand, bridging the gap between research and clinical applications.
As cell-based therapies become mainstream, HPL stands at the forefront, accelerating healing, tissue regeneration, and patient recovery across the globe.
High Production Costs and Scalability Challenges to Hinder Market Growth
Imagine a promising breakthrough in regenerative medicine that could transform patient care, only to be held back by high production costs. While HPL offers a xeno-free, human-derived alternative to traditional cell culture supplements, the process of obtaining, purifying, and standardizing it is labour-intensive and expensive.
Unlike fetal bovine serum (FBS), which benefits from well-established large-scale production, HPL relies on human donor platelets, which can be limited in supply and subject to variability in quality. The industry faces scalability hurdles. As demand for cell-based therapies rises, manufacturers struggle to ramp up production while maintaining consistency and regulatory compliance.
Stringent safety and sterility standards further amplify the challenge, making large-scale, cost-effective manufacturing difficult. Without breakthroughs in cost reduction and process optimization, the high price of HPL may limit its accessibility and widespread adoption, particularly in emerging markets.
Breakthroughs in Bio-manufacturing and Scalable Production to Augment Market Demand
Companies and research institutions are now focusing on advancing production technologies to make HPL more accessible. Efforts are underway to optimize platelet collection, enhance preservation techniques, and develop automated manufacturing systems to ensure consistent, high-quality production.
Advancements in bioreactor technology and closed-system processing could significantly lower production costs, making HPL-based therapies more affordable and widely available.
With governments and private investors supporting bio-manufacturing advancements, the opportunity to bring cost-efficient, large-scale HPL production to the market is closer than ever.
Product Insights
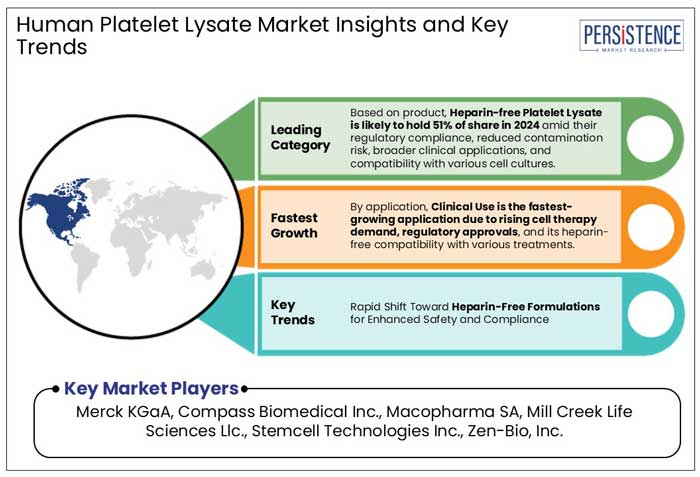
Heparin-Free Human Platelet Lysate (HPL) Leads the Product Segment with 51% Market Share
Heparin-free human platelet lysate (HPL) has emerged as the frontrunner, capturing approximately 51% of the market share. The preference for heparin-free HPL is a reflection of the scientific community's commitment to safety and efficacy. Traditional cell culture supplements often relied on animal-derived components, which posed risks of contamination and variability.
Heparin-free HPL, derived entirely from human platelets without the addition of anticoagulants like heparin, offers a xeno-free alternative that aligns with the stringent requirements of clinical applications.
Researchers have found that heparin-free HPL provides a more consistent and reliable environment for cell growth, particularly for mesenchymal stem cells (MSCs) and T cells. The journey towards adopting heparin-free HPL has been marked by rigorous scientific inquiry and a dedication to improving patient outcomes.
As the field of regenerative medicine continues to advance, the reliance on heparin-free HPL exemplifies a broader shift towards safer, more effective, and ethically sound therapeutic practices.
Application Insights
Clinical use of Human Platelet Lysate (HPL) has Emerged as the Dominant Application, Capturing approximately 94% of the Market
The clinical use of HPL is a testament to its transformative impact on patient care and therapeutic practices. HPL is a solution rich in growth factors and cytokines derived from human platelets.
When applied, it accelerates tissue regeneration, reduces inflammation, and promotes healing where other treatments have failed.
Clinicians across the globe have embraced HPL for its versatility and efficacy. From treating osteoarthritis by enhancing cartilage repair to aiding in the recovery of tendon injuries, HPL has become a cornerstone in various medical specialties.
As the medical community continues to seek advanced solutions for complex health issues, the role of HPL is poised to expand, bringing hope and healing to more patients worldwide.
North America Dominates with 34% Market Share Due to Robust Biotechnology Sector
North America leads the global market with the region's unwavering commitment to innovation and a well-established biotechnology sector, strong R&D investments, and regulatory approvals supporting cell-based therapies.
With a robust biotechnology sector and substantial investments in research and development, North America has become a fertile ground for advancements in cell-based therapies.
The U.S. plays a key role in the region's progress due to the presence of major biopharmaceutical companies and research institutions. This further fuels this momentum, creating an ecosystem where HPL applications thrive, aiding the region's growth over the forecast period.
Asia Pacific to Rapidly Grow Owing to Surge in Healthcare Investments
The growth of the Asia Pacific region is propelled by a surge in healthcare investments and a growing focus on stem cell research.
Nations within this diverse region embrace HPL as a viable alternative to traditional cell culture supplements, driven by the dual imperatives of advancing medical research and addressing unique healthcare challenges. The dynamic economic growth and increasing scientific collaborations in the Asia Pacific are setting the stage for HPL to transform the region's medical landscape.
Europe's Progressive Approach to Healthcare Caters to Region's Substantial Market Share
In the evolving narrative of regenerative medicine, Europe emerges as a pivotal contributor to the Human Platelet Lysate (HPL) market, reflecting a harmonious blend of tradition and innovation.
Europe's growth is underpinned by Europe's rich history in medical research and a progressive approach to healthcare. Substantial investments in research and development evidence the continent's commitment to advancing cell-based therapies.
Europe's stringent regulatory environment fosters a culture of safety and efficacy, encouraging the adoption of HPL as a superior alternative to animal-derived supplements. This regulatory framework ensures patient safety and enhances the credibility of HPL applications in clinical settings.
The Human Platelet Lysate (HPL) Market is becoming increasingly competitive as demand for serum-free, xeno-free growth supplements rises in cell therapy and regenerative medicine.
Key players in the industry are driving innovation with advanced formulations and expanded production capabilities. Startups and biotech firms are also entering the space, focusing on cost-effective and GMP-compliant solutions.
Strategic partnerships, mergers, and acquisitions are shaping the landscape as companies aim to strengthen their global footprint. Regulatory compliance remains a key differentiator, with firms investing in rigorous quality standards to gain an edge. As the market grows, differentiation through product purity, scalability, and ethical sourcing will be critical for sustained success.
Key Industry Developments
The market is set to reach US$ 57.7 Mn in 2025.
Merck KGaA, Compass Biomedical Inc., Macopharma SA, are a few leading players.
The industry is estimated to rise at a CAGR of 3.4% through 2032.
The shelf life of human platelet lysate is estimated to be around seven years.
The market for is anticipated to reach a valuation of US$ 72.5 million by 2032.
|
Report Attributes |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis Units |
Value: US$ Bn/Mn, Volume: As applicable |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Product
By Application
By End User
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author