Industry: Healthcare
Published Date: April-2025
Format: PPT*, PDF, EXCEL
Delivery Timelines: Contact Sales
Number of Pages: 192
Report ID: PMRREP11737
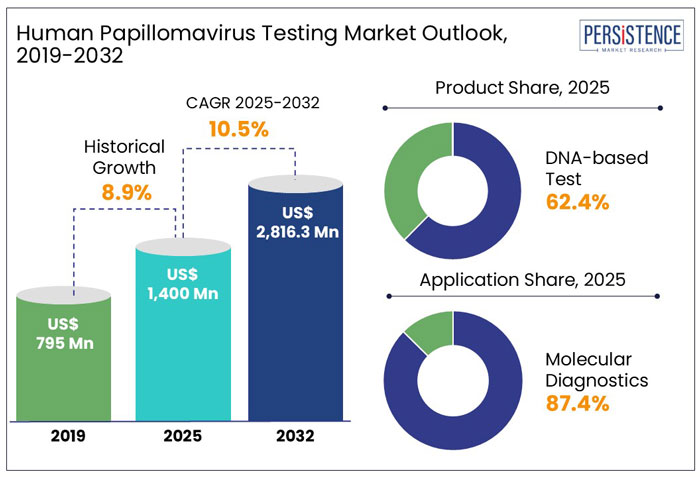
The global human papillomavirus (HPV) testing market size is anticipated to rise from US$ 1,400 Mn in 2025 to US$ 2,816.3 Mn by 2032. It is projected to witness a CAGR of 10.5% from 2025 to 2032.
Human papillomavirus (HPV) testing is a diagnostic procedure used to detect the presence of high-risk HPV strains that can lead to cervical cancer and other HPV-related diseases. The demand for HPV tests globally is experiencing an uptick on account of the increasing cases of cancer and mortality. According to the American Cancer Society, in 2025, approximately 2,041,910 new cancer cases and 618,120 cancer deaths are estimated to occur in the U.S.
It is to be noted that cervical cancer is the fourth most common cancer form in women and needs attention. Global health agencies and national governments are focusing on cervical cancer prevention initiatives through screening, vaccination, and treatment, which is anticipated to favor the market growth through 2032.
As per this Persistence Market Research report, companies such as Abbott Laboratories, Thermo Fischer Scientific Inc., Qiagen N.V., and others are focusing on introducing innovative products, investments in research and development, and leveraging technological advancements to maintain a stronghold in the market.
Key Highlights
|
Global Market Attributes |
Key Insights |
|
Human Papillomavirus Testing (HPV) Market Size (2025E) |
US$ 1,400 Mn |
|
Market Value Forecast (2032F) |
US$ 2,816.3 Mn |
|
Projected Growth (CAGR 2025 to 2032) |
10.5% |
|
Historical Market Growth (CAGR 2019 to 2024) |
8.9% |
Historical Period Witnessed Increasing Number of Screenings in Emerging Economies
In the historical period from 2019 to 2024, the global market for human papillomavirus (HPV) testing showcased a CAGR of 8.9%.
The onset of COVID-19 severely impacted the HPV vaccination programs globally. Since 2019, HPV vaccination coverage dropped at an alarming rate of 15%. This represented one of the most significant drops in any vaccine during the pandemic. Further, as per UNICEF, in 2020, around 600,000 new cervical cancer cases and 340,000 deaths were reported. 90% of these cases and deaths occurred in low and middle-income countries, where people had limited access to prevention, screening, and treatment services.
However, to improve the situation, agencies such as UNICEF focused on working closely and partnering with vaccine manufacturers to provide a steady supply and availability of HPV vaccination in emerging countries. The growing demand for HPV screening and prevention led to a significant improvement in early detection of cases globally. For instance,
Positive Future Outlook: Collaborative Approach by Companies to Develop Innovative Testing Products
The global human papillomavirus (HPV) testing market is likely to showcase a CAGR of 10.5% from 2025 to 2032. Technological advancements in telemedicine and the adoption of collaborative strategies by healthcare companies are likely to boost the market. For instance,
In February 2025, Metropolis Healthcare, one of India’s leading pathology lab chains, collaborated with Roche Diagnostics to introduce the self-sampling HPV DNA test for cervical cancer screening. This collaboration is aimed at improving the early detection of cancer cases through easy access towards the screening process, especially for women living in Tier 2, Tier 3, and Tier 4 cities.
The increasing preference for HPV self-collection testing, which enables collecting samples from any location as per convenience, is empowering women to take control of their health.
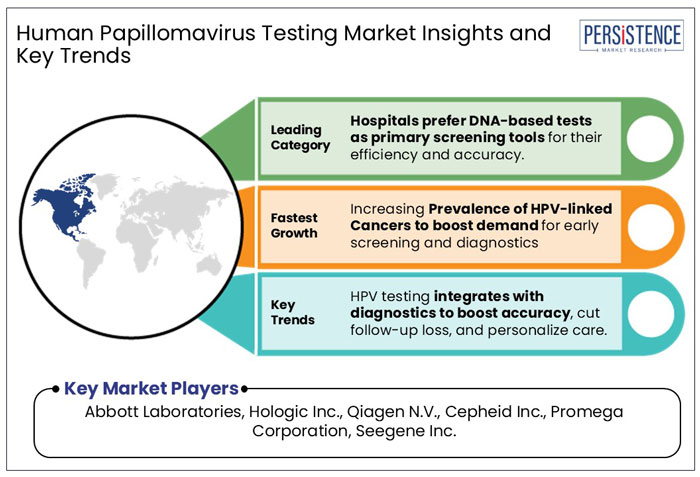
Increasing Prevalence of HPV-Linked Cancers to Boost Demand for Early Screening
HPV is a major cause of cancer globally, including cervical, anal, oropharyngeal, vaginal, penile, and vulvar, among others. Cervical cancer is the most common HPV-associated cancer type among women, and oropharyngeal cancer is the most common among males. According to the U.S. Centers for Disease Control and Prevention (CDC), every year in the United States, around 47,984 new cancer cases are discovered in parts of the body where HPV is commonly found. Of these, 37,800 cancers are caused due to HPV.
The increasing prevalence of HPV-linked cancers is driving the demand for screening, testing, and treatment procedures, which is anticipated to drive market growth during the forecast period.
Frequent Changes in Screening Guidelines to Impede Market Growth
The American Cancer Society’s (ACS) cervical cancer screening guidelines advise commencing HPV testing at age 25 and repeating it every 5 years. Or using a pap test every 3 years, or a combo of HPV/pap test every 5 years. However, frequent revisions to guidelines such as those concerning screening intervals and initiation ages may lead to confusion among healthcare providers and public. Moreover, as vaccination coverage increases, HPV prevalence is expected to decline, initiating further changes to screening guidelines. This factor may affect the demand for HPV testing to a certain extent during the forecast period.
To mitigate the risk of lower screening compliance rates, it is important for policymakers to effectively communicate guidelines and evidence of benefits to women and healthcare workers.
Integration of HPV Testing with Other Diagnostic Tools to Create New Avenues for Market Players
Integrating HPV testing with other diagnostic tools enhances cancer screening by improving accuracy, reducing follow-up losses, and providing customized approaches to different clinical settings. Established companies like Roche Diagnostic, BD, Qiagen, Abbott, and others are focusing on introducing advanced triage options consisting of extended genotyping and dual-stain cytology. For example, Roche Diagnostic’s HPV screening platform helps to detect HPV16, HPV18, and 12 other high-risk HPV specimens.
Approvals for human papillomavirus (HPV) testing procedures by government agencies are further propelling the companies to introduce advanced diagnostic solutions. For instance, the Roche Cobas HPV test is the only HPV test approved by the Food and Drug Administration (FDA) for primary cervical cancer screening in the United States. The test is approved for use with both ThinPrep and SurePath liquid-based cytology specimens.
Growing Preference for Primary HPV Screening to Propel Demand for DNA-based Tests
Based on product, the DNA-based test segment is projected to hold a market share of about 62.4% in 2025. The segment is further forecasted to witness considerable growth, backed by growing preference for HPV DNA testing as a primary screening tool, replacing or supplementing Pap smears in countries such as the U.S., Australia, and others. DNA-based tests such as PCR (Polymerase Chain Reaction) and Hybrid Capture 2 are more reliable than traditional Pap smears in detecting high-risk HPV strains, while reducing false negatives. Further, increasing public awareness campaigns and preventive healthcare initiatives have led to more women opting for DNA-based tests, which is anticipated to drive the segmental growth during the forecast period.
Technological Advancements in PCR & Next-Gen Sequencing (NGS) to Surge Demand for Molecular Diagnostics
On the basis of application, the molecular diagnostics segment is expected to dominate and hold a human papillomavirus (HPV) testing market share of about 87.4% in 2025. The dominance of the segment is attributable to the increasing demand for real-time PCR and NGS technologies due to their accuracy, scalability, and efficiency in HPV testing and other disease detection. For instance, NGS allows parallel sequencing of thousands of DNA fragments, making it ideal for large-scale HPV screening programs.
Availability of Centralized Diagnostic Facilities to Drive Demand for Hospitals Globally
Based on end user, the hospital segment is expected to register a market share of about 53.5% in 2025. The dominance of the segment is attributed to the availability of advanced diagnostic technologies, high patient volumes, comprehensive care offerings, and regulatory support. Hospitals provide both routine screenings and diagnostic tests while managing the treatment and follow-up care of HPV-related conditions.
Availability of Robust Diagnostic Capabilities to Spur Product Demand
North America is projected to hold the major revenue share of 40.3% in the human papillomavirus (HPV) testing industry in 2025. It is likely to maintain its dominance throughout the forecast period.
The availability of robust diagnostic capabilities, particularly through advancements in dual-stain cytology using p16 and Ki-67 markers, is enhancing the efficiency and accuracy of cervical cancer screening. This method is superior to traditional Pap tests for managing HPV-positive results, as it can identify precisely which individuals are at high risk of developing precancerous lesions. In addition, the integration of AI tools like Cytoreader-V2 is expected to improve the process by reducing subjectivity and increasing specificity, which means fewer unnecessary follow-up procedures are required. Apart from technological advancements in diagnostic tools, the increasing adoption of HPV vaccines, such as Cervarix & Gardasil, in North America is projected to propel market growth during the forecast period.
GlaxoSmithKline offers Cervarix free of charge to low-income women in the age group of 19 to 25 without insurance through its Vaccines Access Program. Such initiatives have led to higher adoption of vaccines, which in turn will propel the demand for HPV testing in the region.
Proactive Government Initiatives to Encourage HPV Vaccination to Favor Growth
The market for human papillomavirus (HPV) testing in the Asia Pacific is anticipated to register substantial growth between 2025 and 2032. Factors such as increasing demand for better healthcare infrastructure and supportive government initiatives are expected to boost the demand for HPV testing in countries such as India, China, Korea, and Japan. For example, in April 2025, the Indian government launched a comprehensive training program for doctors to advocate for the HPV vaccine. This initiative focuses on educating communities, countering misinformation, and encouraging discussions about the vaccine’s benefits. Approximately 11,000 members of the Federation of Obstetric and Gynecological Societies of India (FOGSI) underwent virtual training. This effort aligns with the government’s plan to include a domestically produced cervical cancer vaccine in the national immunization program.
Rising Pharmaceutical Industry to Boost Vaccine Development & Ensure Stable Supply Chain
The global market for human papillomavirus is witnessing considerable growth, driven by increasing investments from prominent pharmaceutical companies, strategic collaborations, and supply chain expansion. Major players such as Merck & Co., GlaxoSmithKline, and others are investing heavily in manufacturing sites across Europe to cater to the increasing demand for the HPV vaccine. Merck, for example, has expanded its vaccine production facilities in Austria and the Netherlands. Moreover, several European Union (EU) countries, such as France and Germany, have long-term procurement agreements with Merck to ensure a stable vaccine supply.
Factors such as government collaborations, rising investments from the private-sector, supply chain advancements, and the rising pharma sector are projected to fuel the growth of the market in Europe during the forecast period.
The global market for human papillomavirus (HPV) testing is witnessing intense competition, with key players focusing on product innovations, strategic mergers and acquisitions, and collaborations to strengthen their market presence. Companies are developing high-sensitivity molecular assays, diagnostic tools using AI, and self-sampling kits to enhance screening accuracy and accessibility.
Additionally, partnerships between diagnostic firms and healthcare organizations are expanding testing capabilities in underserved regions. Major players like Roche, BD, Qiagen, and Hologic are investing in automated PCR-based HPV tests and digital cytology platforms to meet the growing demand for early detection and precision diagnostics.
For instance, according to studies, in March 2024, Hologic received FDA approval for its Aptima HPV assay on the Panther Fusion system, enabling faster, high-throughput molecular screening. This development enhances lab efficiency and testing accuracy, reinforcing the shift toward fully automated and integrated HPV diagnostic solutions. As more companies adopt screening using AI and telemedicine-based testing models, the market is set for continuous technological evolution, further driving adoption rates and improving global HPV screening programs.
|
Report Attributes |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis Units |
Value: US$ Bn/Mn, Volume: As applicable |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Product
By Application
By End User
By Region
To know more about delivery timeline for this report Contact Sales

The market is set to reach US$ 1,400 Mn in 2025.
The industry will likely be valued at US$ 2,816 Mn in 2032.
The industry is set to surge at a CAGR of 10.5% through 2032.
Qiagen N.V., Cepheid Inc., and Hologic Inc., are a few key brands.
North America is anticipated to hold the largest share in the market in 2025.