ID: PMRREP34725| 189 Pages | 8 Jul 2025 | Format: PDF, Excel, PPT* | Healthcare

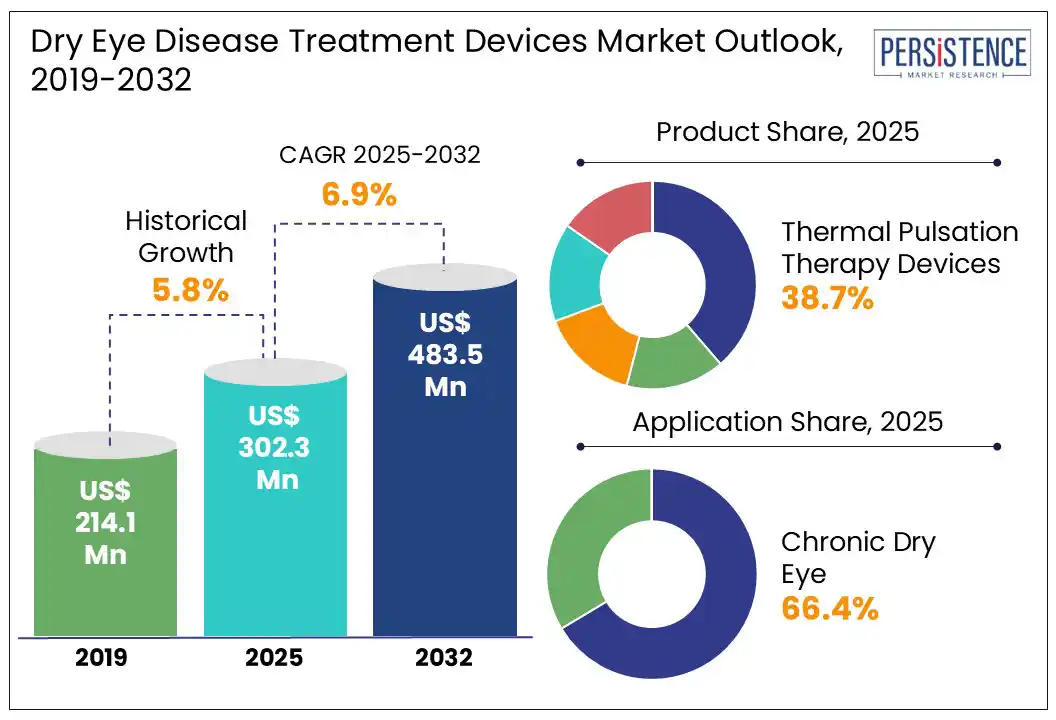
The global dry eye disease treatment devices market size is likely to be valued at US$ 302.3 Mn in 2025, and is expected to reach US$ 483.5 Mn by 2032, growing at a CAGR of 6.9% during the forecast period from 2025 to 2032.
Rising awareness about eye health, advancements in diagnostic and therapeutic technologies, and the growing aging population contribute to driving factors for the global market. Treatment devices such as thermal pulsation systems, intense pulsed light (IPL) devices, neurostimulation tools, and punctal occlusion devices are gaining traction for their ability to provide targeted and effective relief. These innovations improve patient outcomes by addressing underlying causes such as meibomian gland dysfunction (MGD) and inflammation, fuelling market expansion across developed and emerging regions.
Key Industry Highlights:
|
Global Market Attribute |
Key Insights |
|
Dry Eye Disease Treatment Devices Market Size (2025E) |
US$ 302.3 Mn |
|
Market Value Forecast (2032F) |
US$ 483.5 Mn |
|
Projected Growth (CAGR 2025 to 2032) |
6.9% |
|
Historical Market Growth (CAGR 2019 to 2024) |
5.8% |
The global dry eye disease treatment devices market is driven significantly by the growing demand for effective, at-home treatment options that emphasize patient compliance and convenience. Rising awareness of dry eye conditions and the preference for minimally invasive procedures have fuelled innovation in device technology. Technological advances such as real-time imaging personalized therapy protocols, further enhance treatment efficacy and adherence.
For example, Lumibird Medical’s C.STIM™ Intense Pulsed Light (IPL) system launched in February 2022, addresses core issues like Meibomian Gland Dysfunction (MGD) and inflammation using uniform light technology, ensuring effective and comfortable treatment across diverse skin types. Alcon’s Systane iLux2, launched in April 2022, uses thermal pulsation combined with imaging to unblock glands quickly, with results visible within a week.
Additionally, the April 2024 collaboration between Eyenovia and SGN Nanopharma focuses on integrating innovative drug delivery systems with device technology, aiming to improve therapeutic efficacy and patient outcomes worldwide. These advances collectively propel market growth by meeting evolving patient and clinical needs.
The high cost of advanced dry eye treatment devices such as thermal pulsation systems and IPL devices limits their accessibility, especially for lower-income patients or those with inadequate insurance coverage. For example, thermal pulsation device, can cost patients around $500 to $900 per treatment session, IPL therapy ranges from $300 to $700 per session, and punctal plug insertion averages $1,141. These technologies require substantial initial investments, along with ongoing expenses for maintenance, training, and operation, making treatments expensive.
Additionally, treatment efficacy varies widely among patients, influenced by individual characteristics, disease severity, and underlying causes such as MGD or inflammation. This variability, combined with financial barriers, limits widespread adoption and consistent treatment outcomes across diverse patient populations.
The global dry eye disease treatment devices market presents significant opportunities for manufacturers driven by rising prevalence, increasing awareness, and growing demand for innovative, effective therapies. As patients and clinicians seek safer, minimally invasive solutions, companies are capitalizing on developing advanced technologies that enhance treatment precision and patient experience. Expansion into emerging markets with unmet needs further broadens growth potential.
For example, SiFi Medical’s Synfo™ device offers a non-invasive, customizable treatment option targeting inflammation and gland dysfunction, addressing evolving clinical needs. Similarly, in May 2025, Voler Systems transformed a proof-of-concept wearable dry eye device into an FDA-approved medical device by redesigning electronics, developing firmware, and ensuring compliance with medical standards, enabling scalable manufacturing and market readiness.
Additionally, CSI Dry Eye Software (version 6.0 launched in June 2025) developed a cloud-based platform that utilizes proprietary algorithms and machine learning to analyze patient data, identify potential causes of dry eye disease, and recommend personalized treatment options. Continuous innovation and patient-centric designs allow manufacturers to differentiate products, address unmet needs, and capitalize on expanding global market opportunities.
Thermal pulsation therapy devices are projected to capture a dominant revenue share of around 38.7% in 2025 due to their targeted treatment of MGD, a leading cause of dry eye disease. These devices use controlled heat and gentle massage to unblock glands and restore natural oil flow, improving tear film stability and patient comfort.
Compared to IPL, which primarily targets inflammation, thermal pulsation offers a more direct mechanical solution. Neurostimulation devices and punctal occlusion methods, while effective, address symptoms rather than gland function, making thermal pulsation a preferred and growing choice in clinical settings.
The chronic eye disease segment is expected to hold a significant revenue share in the global dry eye disease treatment devices market, accounting for around 66.4% in 2025. This is primarily due to the persistent and long-term nature of conditions such as MGD and chronic dry eye syndrome.
Unlike acute dry eye, which is often temporary and triggered by environmental factors or infections, chronic dry eye requires ongoing management and advanced treatment devices. Patients with chronic conditions frequently need continuous therapies such as thermal pulsation or punctal plugs, driving higher demand and revenue in this segment compared to the short-term, less device-intensive acute dry eye cases.
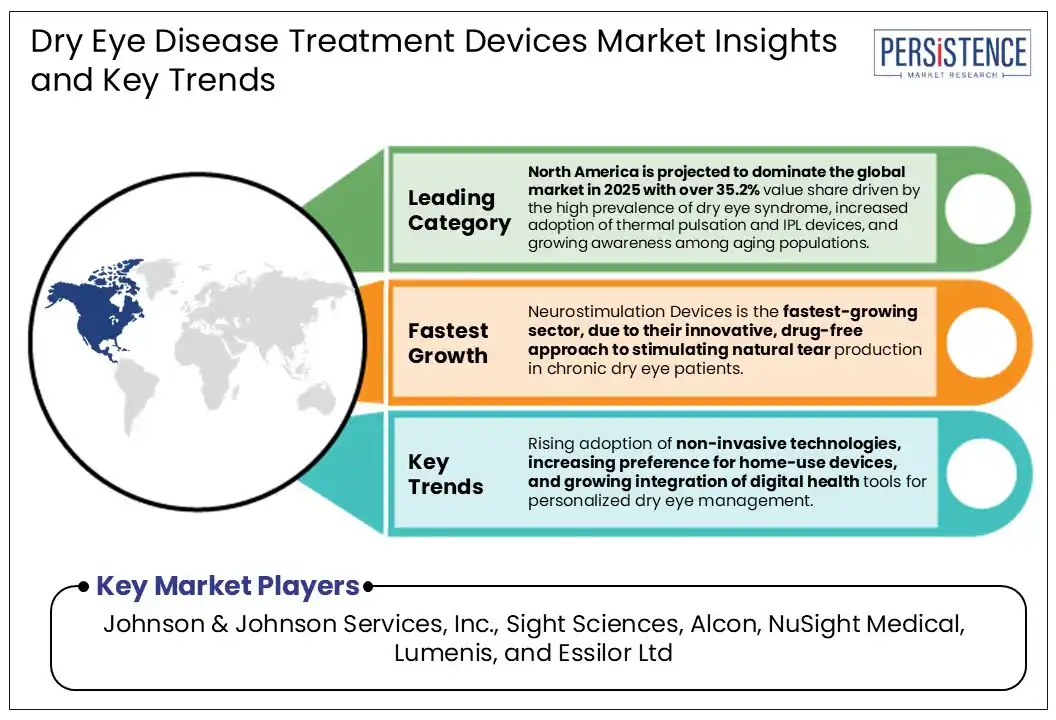
North America is anticipated to dominate the global market in 2025 with 38.7% value share driven by the high prevalence of dry eye syndrome, increased adoption of thermal pulsation and IPL devices, and growing awareness among aging populations.
The U.S. dry eye disease treatment devices market, in particular, benefits from strategic partnerships and product launches that enhance diagnostic precision and therapeutic options. For example, in December 2023, Reichert Technologies partnered exclusively with SBM Sistemi to distribute advanced devices like the IDRA and OS1000, improving ocular surface assessment. In 2024, Reichert further expanded its portfolio with four new devices, including the Activa, targeting MGD with controlled heating and micro-vibrations.
Additionally, in June 2024 Nordic Pharma launched Lacrifill, a canalicular gel device shown to improve tear retention, while Novoxel’s thermal technology based Tixel device received U.S.FDA clearance in December 2024, for treating evaporative dry eye. These innovations and regulatory support position North America as the market leader.
Europe is emerging as a key region in the global market, driven by increasing health awareness, advanced clinical infrastructure, and strong regulatory support for innovation. Countries such as Germany, France, and the UK are fostering rapid adoption of non-invasive and tech-enabled solutions. This environment is also nurturing a wave of innovation and startups focused on digital therapeutics, wearable technologies, and personalized eye care.
For instance, in June 2024, Italy-based Espansione Group received CE Mark approval for its eye-light® photobiomodulation device, expanding its use beyond dry eye to include Age-Related Macular Degeneration (AMD) and anterior segment conditions. Additionally, a Cyprus-based startup is also pioneering wearable solutions to monitor and alleviate dry eye symptoms, highlighting the region’s focus on digital health integration.
Furthermore, companies such as Eurotech Optical are offering a broad range of in-clinic and at-home dry eye treatments, emphasizing accessibility and personalization. Europe’s emphasis on research and development and early adoption of innovative technologies continues to strengthen its position in the global market.
Asia Pacific market is rapidly expanding, driven by increasing awareness, rising healthcare investment, and a high burden of dry eye conditions particularly in densely populated countries. In China alone, the incidence of dry eye disease (DED) ranges from 21% to 30%, second only to myopia, with nearly 400 million affected individuals by the end of 2023 (National Bureau of Statistics of China, 2023). Furthermore, recent studies indicate the incidence of DED in China to be as high as 32.1%, and between 5% and 50% of people over 50 years of age suffer from DED in Asia. This growing patient population is fuelling demand for accessible, non-invasive treatment options.
In June 2024, Zhaoke Ophthalmology entered an exclusive agreement with EyeDtec Medical to commercialize the Eye Lipid Mobilizer, a device designed to improve lipid layer function and enhance outcomes. Similarly, in 2025, China Pharma Holdings plans to launch a novel therapeutic device, through its subsidiary, Hainan Helpson Medical and Biotechnology Co., tailored to this need, offering effective treatment for dry eye patients. These developments highlight the strong growth potential in the China dry eye disease treatment devices market and position Asia Pacific as a key contributor to global market expansion.
Collaborations and partnerships to develop innovative products and accelerate the grant by regulatory bodies are the key growth strategies followed by players in the global dry eye disease treatment devices market. Companies are continuously investing in R&D to introduce innovative and breakthrough dry eye disease treatment devices.
The global dry eye disease treatment devices market is set to reach US$ 302.3 Mn in 2025.
The market is projected to record a CAGR of 6.9% during the forecast period from 2025 to 2032.
The rising prevalence of dry eye syndrome and the growing demand for non-pharmacological, device-based therapies are driving the global market.
Johnson & Johnson Services, Inc., Sight Sciences, Alcon, NuSight Medical, Lumenis, and Essilor Ltd are a few leading players.
North America is projected to dominate the global market in 2025.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Units |
Value: US$ Mn/Bn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Product
By Application
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author