- Executive Summary
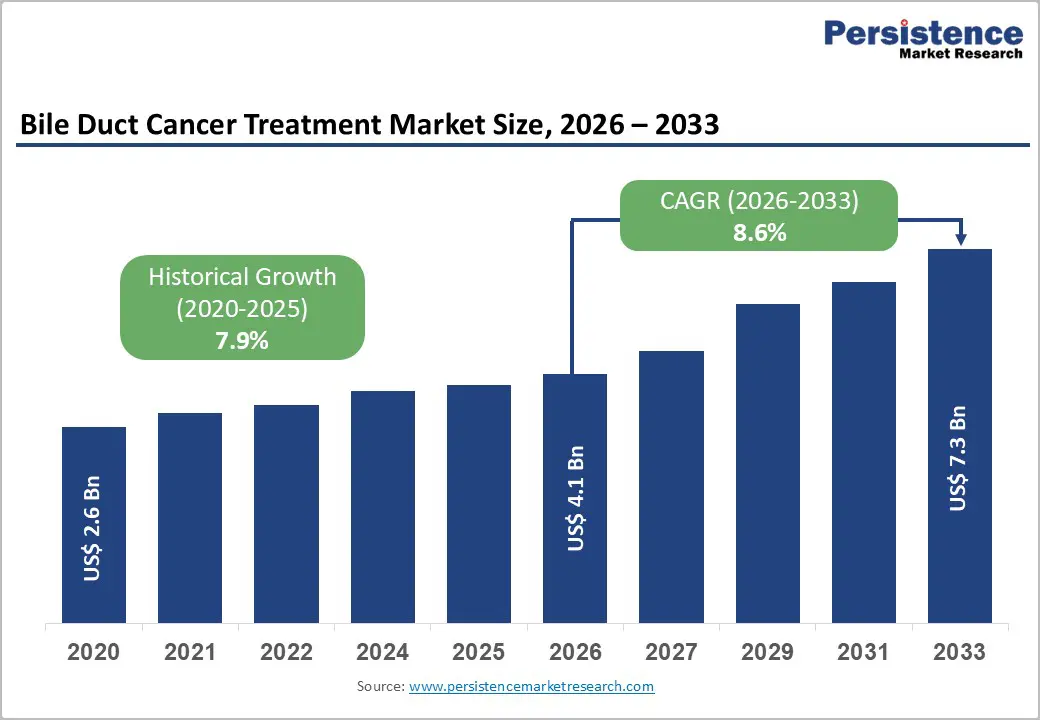
- Global Bile Duct Cancer Treatment Market Snapshot 2026 and 2033
- Market Opportunity Assessment, 2026-2033, US$ Bn
- Key Market Trends
- Industry Developments and Key Market Events
- Demand Side and Supply Side Analysis
- PMR Analysis and Recommendations
- Market Overview
- Market Scope and Definitions
- Market Dynamics
- Driver
- Restraint
- Opportunities
- Trends
- Macro-Economic Factors
- Global GDP Outlook
- Global Healthcare Expenditure
- Forecast Factors - Relevance and Impact
- COVID-19 Impact Assessment
- Value Added Insights
- Value Chain analysis
- Key Market Players
- Product Adoption Analysis
- Unmet Needs
- Pipeline Assessment
- Key Promotional Strategies by key players
- PESTLE Analysis
- Porter's Five Forces Analysis
- Regulatory and Technology Landscape
- Global Bile Duct Cancer Treatment Market Outlook: Historical (2020 - 2025) and Forecast (2026 - 2033)
- Key Highlights
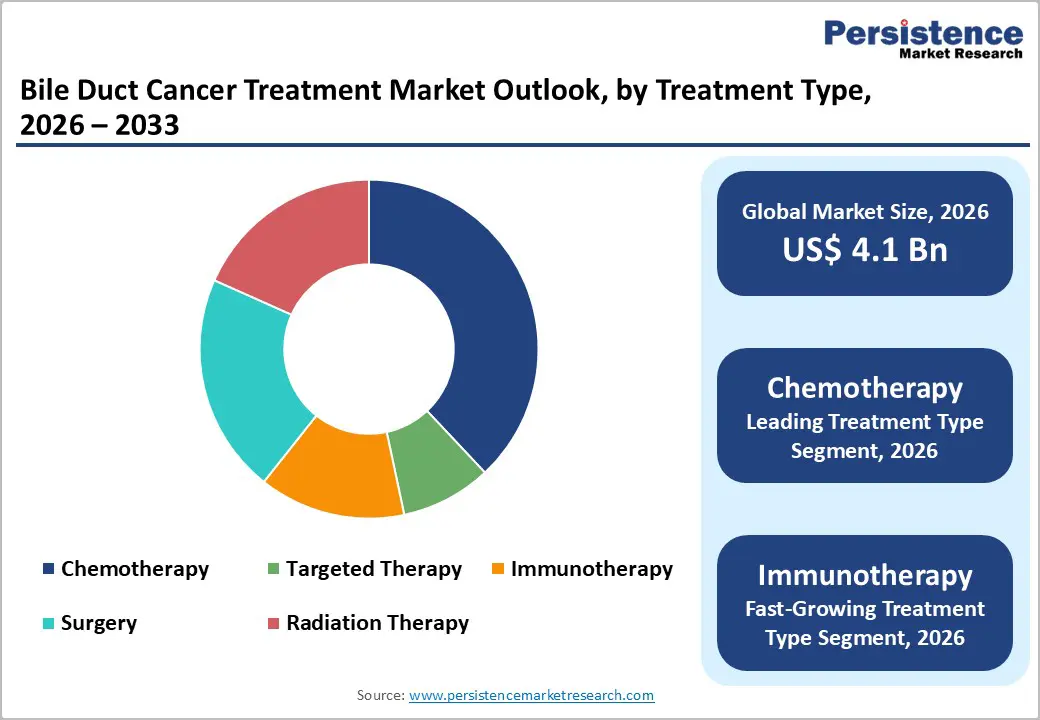
- Global Bile Duct Cancer Treatment Market Outlook: Treatment Type
- Introduction/Key Findings
- Historical Market Size (US$ Bn) Analysis by Treatment Type, 2020-2025
- Current Market Size (US$ Bn) Forecast, by Treatment Type, 2026-2033
- Chemotherapy
- Targeted Therapy
- Immunotherapy
- Surgery
- Radiation Therapy
- Market Attractiveness Analysis: Treatment Type
- Global Bile Duct Cancer Treatment Market Outlook: Cancer Type
- Introduction/Key Findings
- Historical Market Size (US$ Bn) Analysis by Cancer Type, 2020-2025
- Current Market Size (US$ Bn) Forecast, by Cancer Type, 2026-2033
- Intrahepatic bile duct cancer
- Extrahepatic bile duct cancer
- Perihilar Bile Duct Cancer
- Distal Extrahepatic Bile Duct Cancer
- Market Attractiveness Analysis: Cancer Type
- Global Bile Duct Cancer Treatment Market Outlook: Treatment Provider
- Introduction/Key Findings
- Historical Market Size (US$ Bn) Analysis by Treatment Provider, 2020-2025
- Current Market Size (US$ Bn) Forecast, by Treatment Provider, 2026-2033
- Hospitals
- Cancer specialty centers
- Ambulatory care centers
- Market Attractiveness Analysis: Treatment Provider
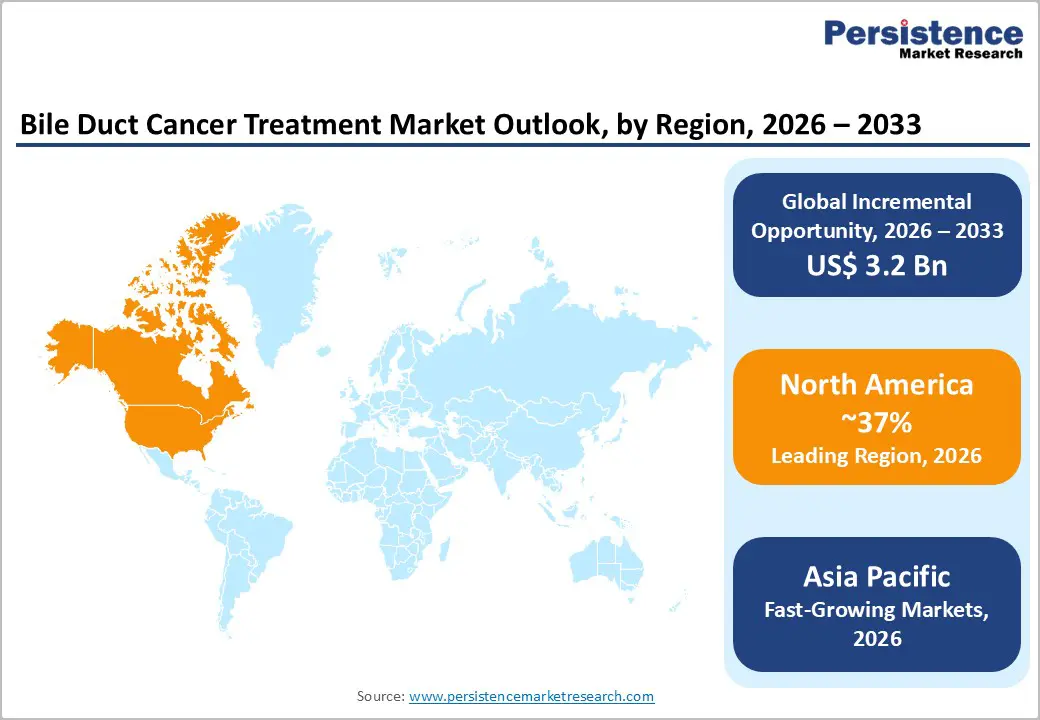
- Global Bile Duct Cancer Treatment Market Outlook: Region
- Key Highlights
- Historical Market Size (US$ Bn) Analysis by Region, 2020-2025
- Current Market Size (US$ Bn) Forecast, by Region, 2026-2033
- North America
- Europe
- East Asia
- South Asia & Oceania
- Latin America
- Middle East & Africa
- Market Attractiveness Analysis: Region
- North America Bile Duct Cancer Treatment Market Outlook: Historical (2020 - 2025) and Forecast (2026 - 2033)
- Key Highlights
- Pricing Analysis
- North America Market Size (US$ Bn) Forecast, by Country, 2026-2033
- U.S.
- Canada
- North America Market Size (US$ Bn) Forecast, by Treatment Type, 2026-2033
- Chemotherapy
- Targeted Therapy
- Immunotherapy
- Surgery
- Radiation Therapy
- North America Market Size (US$ Bn) Forecast, by Cancer Type, 2026-2033
- Intrahepatic bile duct cancer
- Extrahepatic bile duct cancer
- Perihilar Bile Duct Cancer
- Distal Extrahepatic Bile Duct Cancer
- North America Market Size (US$ Bn) Forecast, by Treatment Provider, 2026-2033
- Hospitals
- Cancer specialty centers
- Ambulatory care centers
- Europe Bile Duct Cancer Treatment Market Outlook: Historical (2020 - 2025) and Forecast (2026 - 2033)
- Key Highlights
- Pricing Analysis
- Europe Market Size (US$ Bn) Forecast, by Country, 2026-2033
- Germany
- Italy
- France
- U.K.
- Spain
- Russia
- Rest of Europe
- Europe Market Size (US$ Bn) Forecast, by Treatment Type, 2026-2033
- Chemotherapy
- Targeted Therapy
- Immunotherapy
- Surgery
- Radiation Therapy
- Europe Market Size (US$ Bn) Forecast, by Cancer Type, 2026-2033
- Intrahepatic bile duct cancer
- Extrahepatic bile duct cancer
- Perihilar Bile Duct Cancer
- Distal Extrahepatic Bile Duct Cancer
- Europe Market Size (US$ Bn) Forecast, by Treatment Provider, 2026-2033
- Hospitals
- Cancer specialty centers
- Ambulatory care centers
- East Asia Bile Duct Cancer Treatment Market Outlook: Historical (2020 - 2025) and Forecast (2026 - 2033)
- Key Highlights
- Pricing Analysis
- East Asia Market Size (US$ Bn) Forecast, by Country, 2026-2033
- China
- Japan
- South Korea
- East Asia Market Size (US$ Bn) Forecast, by Treatment Type, 2026-2033
- Chemotherapy
- Targeted Therapy
- Immunotherapy
- Surgery
- Radiation Therapy
- East Asia Market Size (US$ Bn) Forecast, by Cancer Type, 2026-2033
- Intrahepatic bile duct cancer
- Extrahepatic bile duct cancer
- Perihilar Bile Duct Cancer
- Distal Extrahepatic Bile Duct Cancer
- East Asia Market Size (US$ Bn) Forecast, by Treatment Provider, 2026-2033
- Hospitals
- Cancer specialty centers
- Ambulatory care centers
- South Asia & Oceania Bile Duct Cancer Treatment Market Outlook: Historical (2020 - 2025) and Forecast (2026 - 2033)
- Key Highlights
- Pricing Analysis
- South Asia & Oceania Market Size (US$ Bn) Forecast, by Country, 2026-2033
- India
- Southeast Asia
- ANZ
- Rest of SAO
- South Asia & Oceania Market Size (US$ Bn) Forecast, by Treatment Type, 2026-2033
- Chemotherapy
- Targeted Therapy
- Immunotherapy
- Surgery
- Radiation Therapy
- South Asia & Oceania Market Size (US$ Bn) Forecast, by Cancer Type, 2026-2033
- Intrahepatic bile duct cancer
- Extrahepatic bile duct cancer
- Perihilar Bile Duct Cancer
- Distal Extrahepatic Bile Duct Cancer
- South Asia & Oceania Market Size (US$ Bn) Forecast, by Treatment Provider, 2026-2033
- Hospitals
- Cancer specialty centers
- Ambulatory care centers
- Latin America Bile Duct Cancer Treatment Market Outlook: Historical (2020 - 2025) and Forecast (2026 - 2033)
- Key Highlights
- Pricing Analysis
- Latin America Market Size (US$ Bn) Forecast, by Country, 2026-2033
- Brazil
- Mexico
- Rest of LATAM
- Latin America Market Size (US$ Bn) Forecast, by Treatment Type, 2026-2033
- Chemotherapy
- Targeted Therapy
- Immunotherapy
- Surgery
- Radiation Therapy
- Latin America Market Size (US$ Bn) Forecast, by Cancer Type, 2026-2033
- Intrahepatic bile duct cancer
- Extrahepatic bile duct cancer
- Perihilar Bile Duct Cancer
- Distal Extrahepatic Bile Duct Cancer
- Latin America Market Size (US$ Bn) Forecast, by Treatment Provider, 2026-2033
- Hospitals
- Cancer specialty centers
- Ambulatory care centers
- Middle East & Africa Bile Duct Cancer Treatment Market Outlook: Historical (2020 - 2025) and Forecast (2026 - 2033)
- Key Highlights
- Pricing Analysis
- Middle East & Africa Market Size (US$ Bn) Forecast, by Country, 2026-2033
- GCC Countries
- South Africa
- Northern Africa
- Rest of MEA
- Middle East & Africa Market Size (US$ Bn) Forecast, by Treatment Type, 2026-2033
- Chemotherapy
- Targeted Therapy
- Immunotherapy
- Surgery
- Radiation Therapy
- Middle East & Africa Market Size (US$ Bn) Forecast, by Cancer Type, 2026-2033
- Intrahepatic bile duct cancer
- Extrahepatic bile duct cancer
- Perihilar Bile Duct Cancer
- Distal Extrahepatic Bile Duct Cancer
- Middle East & Africa Market Size (US$ Bn) Forecast, by Treatment Provider, 2026-2033
- Hospitals
- Cancer specialty centers
- Ambulatory care centers
- Competition Landscape
- Market Share Analysis, 2025
- Market Structure
- Competition Intensity Mapping
- Competition Dashboard
- Company Profiles
- Merck & Co., Inc.
- Company Overview
- Product Portfolio/Offerings
- Key Financials
- SWOT Analysis
- Company Strategy and Key Developments
- AstraZeneca PLC
- Incyte Corporation
- Taiho Pharmaceutical
- F. Hoffmann-La Roche Ltd.
- Eisai Co., Ltd.
- Pfizer Inc.
- Bristol-Myers Squibb
- Servier Pharmaceuticals
- Agios Pharmaceuticals
- Zymeworks Inc.
- BeiGene, Ltd.
- Merck & Co., Inc.
- Appendix
- Research Methodology
- Research Assumptions
- Acronyms and Abbreviations

Loading page data
Please wait a moment