Industry: Healthcare
Published Date: February-2025
Format: PPT*, PDF, EXCEL
Delivery Timelines: Contact Sales
Number of Pages: 192
Report ID: PMRREP35097
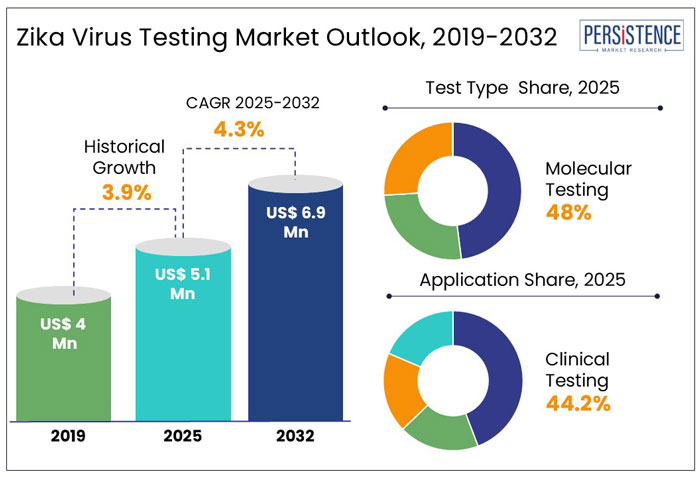
The global zika virus testing market is projected to grow attain a size of US$ 5.1 million in 2025. The industry is anticipated to exhibit a CAGR of 4.3% through 2032 to reach a value of US$ 6.9 million in 2032.
Growth in the industry is primarily driven by the increasing prevalence of zika virus outbreaks fostering a demand rapid and accurate testing solutions to control virus spread. The market for high-quality zika virus testing is being bolstered by elevated awareness of infectious disease management and public health initiatives targeting virus containment. Advancements in testing technology, like point-of-care molecular diagnostics and serological tests, are estimated to accelerate adoption among healthcare providers and public health organizations.
As governments and healthcare systems continue to emphasize disease prevention and early detection, manufacturers in the zika virus testing industry are likely to witness significant growth. The focus on developing effective and accessible testing methods is likely to drive substantial expansion throughout the forecast period.
Key Highlights of the Market
|
Market Attributes |
Key Insights |
|
Zika Virus Testing Market Size (2025E) |
US$ 5.1 Mn |
|
Projected Market Value (2032F) |
US$ 6.9 Mn |
|
Global Market Growth Rate (CAGR 2025 to 2032) |
4.3% |
|
Historical Market Growth Rate (CAGR 2018 to 2023) |
3.9% |
Zika testing market in North America is predicted to hold a share of 42.4% 2025. Growth in the region is predicted to be driven by a well-established healthcare infrastructure and a strong focus on infectious disease management. Population in the region is increasingly becoming aware of zika virus risks, fueling growth in zika virus testing.
North America has an extensive network of hospitals, diagnostic laboratories, and public health organizations that increase the accessibility to a wide range of Zika virus testing options. This includes rapid diagnostic tests to advanced molecular testing solutions.
Widespread adoption of digital health and e-commerce platforms enables healthcare providers to efficiently order testing kits and equipment, supporting faster delivery and broad distribution across the region.
Molecular testing is predicted to hold a share of 48% in 2025. RT-PCR is predicted to emerge as the leading molecular testing. is expected to dominate the zika virus testing market by test type. This growth is driven by high sensitivity and accuracy of molecular tests, which are essential for early detection of zika virus.
RT-PCR, a key molecular test, is widely regarded as the gold standard for zika virus identification as it can detect viral RNA even in the early stages of infection. This enables timely intervention and containment measures.
The demand for other nucleic acid amplification tests (NAATs) is on the rise, as healthcare facilities seek reliable and rapid solutions for zika virus diagnosis. This investment in innovative molecular and serological testing technologies highlights the focus on improving diagnostic accuracy and efficiency, ensuring access to cutting-edge tools to combat zika virus outbreaks.
Clinical testing is anticipated to hold a share of 44.2% in 2025. This dominance is primarily driven by the urgent need for rapid and accurate diagnostics in clinical settings to effectively manage and control zika virus outbreaks. Clinical testing presents healthcare providers with reliable and on-the-spot results. This is essential for diagnosing and treating zika infections, especially in areas where the virus poses a significant threat to public health.
Early detection through clinical testing enables timely patient care, assists in preventing virus spread, and supports effective containment strategies during outbreak situations. During recent outbreaks, hospitals and clinics relied heavily on rapid diagnostic tests (RDTs) and molecular tests to quickly identify and isolate zika cases. This underscores the importance of accessible clinical testing in high-risk regions.
The convenience, efficiency, and broad applicability of zika virus tests in clinical environments make this segment crucial for public health response. As healthcare systems continue to prioritize infectious disease control, the demand for clinical testing in zika virus diagnostics is estimated to remain robust.
Hospitals and clinics are anticipated to hold a share of 49% 2025. These have advanced laboratory infrastructure and trained medical professionals, enabling accurate and timely zika virus testing.
During the 2015 to 2016 zika epidemic, hospitals and clinics in affected regions reported a 25%to 40% increase in visits from individuals with suspected symptoms like fever, rash, and joint pain. Zika virus poses severe risks to pregnant women, including birth defects like microcephaly.
Increase in global awareness of mosquito-borne diseases and their impact on public health has put considerable attention on the zika virus testing market. The zika virus has since become a significant health concern, especially after major outbreaks in South and Central America, Southeast Asia, and parts of Africa. The virus can lead to severe complications, driving a pressing need for accurate and rapid testing solutions to facilitate early detection, timely treatment, and effective containment of outbreaks.
The shift toward molecular diagnostic tools like RT-PCR and other NAATs, offer high sensitivity and specificity for early detection. Growing interest in multiplex testing solutions, enable simultaneous screening for multiple mosquito-borne viruses, which often co-circulate with Zika in affected regions.
In response to rising demand, diagnostic companies are actively developing advanced point-of-care tests. These tests can be used in resource-limited settings, where Zika outbreaks are prevalent and access to healthcare facilities may be restricted.
The zika virus testing market growth was steady at a CAGR of 3.9% during the historical period. Expansion was prominently attributed to rising public health concerns and increased zika cases in vulnerable regions.
Key diagnostic companies made heavy investments in research and development to improve the accuracy, speed, and accessibility of testing. The COVID-19 pandemic further highlighted the need for rapid diagnostic solutions.
Looking ahead, the demand for reliable diagnostic tools in mosquito-borne disease-prone areas is likely to foster demand. Advancements in molecular testing and multiplex diagnostics is likely to fuel expansion in the zika virus testing market.
Enhanced Diagnostic Technologies
Zika virus testing market is witnessing expansion owing to technological advancements, which make diagnostics quicker, accurate, and dependable.
This is essential for differentiating zika from other flaviviruses, like dengue, which can cause diagnostic cross-reactivity and frequently exhibit identical symptoms. Automation features of platforms like the Cobas e immunoassay analyzers enable healthcare institutions to process tests effectively for rapid and extensive testing during epidemics. Improvements in diagnostic tests assists health practitioners to easily handle and respond to zika virus cases, increasing accessibility and accuracy, especially in high-risk locations.
Government Funding and Public Health Initiatives
The 2015 Zika pandemic caused serious birth abnormalities in infected fetuses and required immediate federal action.
Financing from government organizations facilitated investments in research activities and mosquito control. These heavy investments enabled the launch of public awareness programs regarding Zika hazards and prevention.
Government funding in zika virus testing contributed to enhancing the infrastructure for laboratory testing. Florida used monies to improve Zika testing at state-run labs.
Limited Awareness and Access to Information in Low-Income Regions
Effective prevention and control of the zika virus are severely hampered in low-income areas owing to lack of knowledge and access to reliable information. Studies reveal that several pregnant women lack thorough understanding about the virus's transmission and its hazards, including fetal defects, despite extensive media coverage.
The Latino community is particularly at risk as it regularly visits areas where Zika outbreaks are still occurring. Despite the CDC's guidelines, only a small portion of the community is knowledgeable with Zika with 24% of Americans stating they have very little knowledge of the virus.
The issue is made worse by obstacles to information access, including socioeconomic status, language barriers, and restricted access to healthcare. Even if medical professionals are reliable information sources, research shows that public health emergency communication tactics need to be strengthened. Low-income groups continue to be more vulnerable to zika infection and its related problems in the absence of focused educational programs and improved access to reliable information.
Integration with Broader Mosquito-Borne Disease Testing
As diseases like dengue, chikungunya, and Zika share common vectors, integrating diagnostic tools to detect multiple viruses can streamline testing processes and enhance diagnostic efficiency. This approach not only decreases healthcare costs while increasing the accessibility and convenience of testing, especially in regions prone to mosquito-borne outbreaks.
Advances in multiplex testing technologies that can detect co-circulating viruses are estimated to witness high demand. This enables healthcare providers to address multiple public health concerns with a single test. This integration can enhance epidemic preparedness, decrease diagnostic delays, and ultimately strengthen disease control efforts in endemic regions.
Advancing Zika Virus Management with Research and Development Activities
The World Health Organization (WHO), in collaboration with global experts and institutions like CIDRAP and the University of Texas Medical Branch, has prioritized the development of countermeasures against Zika virus. The 10-year roadmap outlines key goals in advancing diagnostics, therapeutics, and vaccines.
As vaccine and therapeutic solutions evolve, there will be an increased need for accurate and reliable diagnostic tests to support treatment and vaccination efforts. This alignment between diagnostic and therapeutic advancements can stimulate investment in both areas, leading to better healthcare responses to outbreaks.
The roadmap's emphasis on addressing knowledge gaps and encouraging collaboration is likely to accelerate breakthroughs in the research and development of zika virus. This ensures comprehensive tools for disease control while improving public health preparedness globally.
The global zika virus testing market is highly competitive, with large and small companies developing innovative molecular and serological testing solutions. Prominent players focus on improving testing accuracy, speed, and accessibility, while local manufacturers increase market presence through online platforms and health events.
Emerging startups, supported by funding and exposure from industry networks, are predicted to bring cutting-edge diagnostic solutions. This dynamic environment is driving continuous growth and innovation in zika virus testing technologies across the globe.
Recent Developments in the Zika Virus Testing Market
|
Attributes |
Details |
|
Forecast Period |
2025 to 2032 |
|
Historical Data Available for |
2019 to 2023 |
|
Market Analysis |
US$ Million for Value |
|
Key Regions Covered |
|
|
Key Market Segments Covered |
|
|
Key Companies Profiled in the Report |
|
|
Report Coverage |
|
|
Customization & Pricing |
Available upon request |
By Test Type
By Application
By End Use
By Region
To know more about delivery timeline for this report Contact Sales

The industry is projected to reach US$ 6.9 million in 2032.
Key consumers include hospitals, diagnostic labs, public health organizations, and research institutes.
India is estimated to capture a significant share by 2032 owing to increased awareness and public health initiatives.
Roche Diagnostics, Abbott Laboratories, and Cepheid are leading players, known for their innovative testing solutions.
Rising investments by government bodies in infectious disease prevention are projected to open the door to opportunities.