U.S. Polycystic Ovarian Syndrome Treatment Market Segmented By Oral Contraceptives, Insulin Sensitizing Agents, Anti-Depressants, Ornithine, Decarboxylase Inhibitors, Aromatase Inhibitors and SERMs, Diuretics Drug Class
Industry: Healthcare
Published Date: March-2017
Format: PPT*, PDF, EXCEL
Delivery Timelines: Contact Sales
Number of Pages: 70
Report ID: PMRREP14317
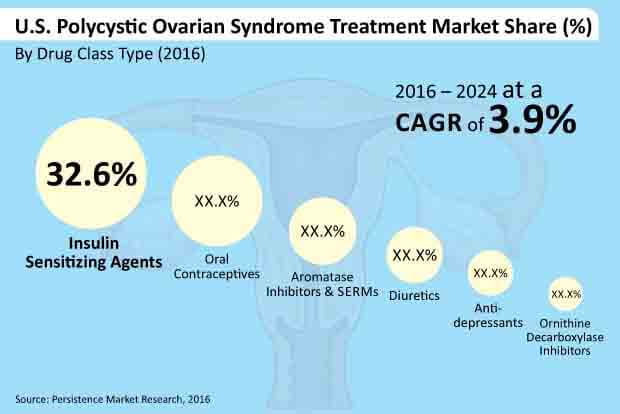
The insulin sensitizing agents drugs class segment is estimated to lead in terms of revenue contribution to the U.S. polycystic ovarian syndrome treatment market and is estimated to account for a little more than 30% market share by the end of 2024. The segment is likely to be valued at over US$ 75 Mn in 2017 and is expected to reach close to US$ 100 Mn by 2024 end. Currently, around 70% of polycystic ovarian syndrome patients are overweight and prone to type 2 diabetes.
Thus, insulin sensitizing agents are the most widely prescribed drugs for polycystic ovarian syndrome treatment. Insulin sensitizing agents drug class segment is expected to reflect a market attractiveness index of 2.0 over the estimated period. The segment is anticipated to create absolute $ opportunity of close to US$ 3 Mn in 2017 over 2016.
Increasing healthcare expenditure is being witnessed in many developed countries worldwide including the U.S. This indicates that people have become more health conscious and hence are increasingly inclined towards enhanced treatment options for various ailments. This is one among the primary factors driving the growth of the insulin sensitizing agents drug class segment. Polycystic ovarian syndrome associated diseases, especially obesity and insulin insensitivity are increasing; however, the exact cause of polycystic ovarian syndrome is so far unknown and there are no drugs approved for direct treatment of polycystic ovarian syndrome.
However, associated conditions can be controlled with drugs such as insulin sensitizing agents. Thus, an increasing need for associated disease management is expected to contribute significantly to the growth of the insulin sensitizing agents drug class segment. Also, rising R&D expenditure and an introduction of novel drugs is expected to fuel market growth of the insulin sensitizing agents drug class segment over the forecast period. Companies in the U.S are currently focusing on research and development of novel drugs to enable better management of associated diseases such as obesity, insulin sensitivity, hirsutism etc.
Besides, polycystic ovarian syndrome drugs manufacturers are focusing on the commercial distribution of medicines through retail pharmacies and online portals. Online portals widely distribute polycystic ovarian syndrome drugs with or without prescription. Moreover, consumers can avail discounts on bulk purchase of medicines through online portals.
Hence, easy availability and discounts contribute significantly to increasing adoption of insulin sensitizing agents. The development of the insulin sensitizing agents drug class segment is also attributed to increasing disposable income and improving healthcare facilities in the U.S. The growth of the segment is further credited to several awareness programs initiated by the U.S government.
Drugs used for polycystic ovarian syndrome are not covered in most coverage policies or health insurance. The cost of few drugs are comparatively high in the U.S. Thus, reduction in the cost of branded drugs would help in the adoption of polycystic ovarian syndrome drugs across the globe.
Currently, only Metformin, an insulin sensitizing agent has been included in the list of reimbursed medicines. Taking into consideration the high cost of insulin sensitizing agents in various countries such as the U.S., polycystic ovarian syndrome drugs could be covered under reimbursement policies, which would help propel the growth of the insulin sensitizing agents drug class segment.
Players in the U.S polycystic ovarian syndrome treatment market are likely to benefit through higher investment by government authorities and funding agencies for the development of new drugs for the treatment of polycystic ovarian syndrome.
Currently, there are no drugs approved by FDA and EMA for polycystic ovarian syndrome, thus drugs manufacturers are being presented an opportunity to hold significant share in the market. In addition, the patient population of diabetes is expected to increase at a high rate due to a sedentary lifestyle. As a result, the demand for branded drugs is expected to increase significantly over the predicted period.
|
By Drug Class |
|
|
By Distribution Channel |
|
To know more about delivery timeline for this report Contact Sales
