Industry: Healthcare
Published Date: April-2025
Format: PPT*, PDF, EXCEL
Delivery Timelines: Contact Sales
Number of Pages: 180
Report ID: PMRREP14305
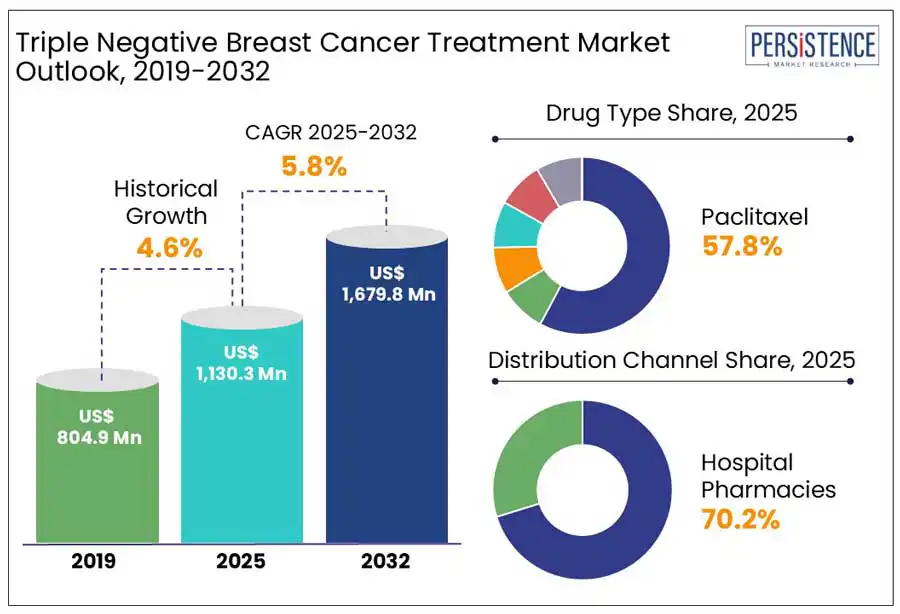
The global triple negative breast cancer treatment market size is projected to rise from US$ 1,130.3 Mn in 2025 to US$ 1,679.8 Mn by 2032. It is anticipated to exhibit a CAGR of 5.8% during the forecast period from 2025 to 2032.
The poor prognosis and aggressive nature of triple negative breast cancer (TNBC) is anticipated to spur demand for intensive and early treatment. Studies revealed that TNBC patients have a high risk of recurrence in the first three years after diagnosis. Metastasis often occurs in significant organs such as brain, liver, and lungs. It will likely propel demand for innovative treatment options, including PARP inhibitors and immunotherapy.
Key Industry Highlights
|
Global Market Attributes |
Key Insights |
|
Triple Negative Breast Cancer Treatment Market Size (2025E) |
US$ 1,130.3 Mn |
|
Market Value Forecast (2032F) |
US$ 1,679.8 Mn |
|
Projected Growth (CAGR 2025 to 2032) |
5.8% |
|
Historical Market Growth (CAGR 2019 to 2024) |
4.6% |
TNBC is not susceptible to hormone therapy or HER2-targeted medications, which work well for other forms of breast cancer, as it lacks estrogen, progesterone, and HER2 receptors. This is set to propel the need for novel and innovative treatment approaches, fueling the market. Investment in immunotherapy-based solutions has increased due to the approval of immune checkpoint inhibitors such as Atezolizumab (Tecentriq) in combination with chemotherapy.
Increasing prevalence of TNBC, especially among younger women is likely to augment the need for effective treatment options. The American Cancer Society found that compared to white women, African American women are twice as likely to receive a TNBC diagnosis. Research into targeted treatments such as Sacituzumab Govitecan (Trodelvy), an antibody-drug combination that has shown notable success in patients with metastatic TNBC, has been boosted by this demographic disparity.
Demand for TNBC treatment is set to decline to a certain extent due to a shift toward early detection and prevention measures. High-risk individuals, mainly those with Breast Cancer (BRCA) gene mutations, are likely to incline toward prophylactic mastectomy, a preventive approach due to rising awareness of risk-reduction strategies and genetic screening. For example, the number of late-stage TNBC diagnoses and the need for therapy in such cases decreased due to actress Angelina Jolie's prophylactic double mastectomy after testing positive for the BRCA1 gene mutation. Also, owing to severe toxicity of chemotherapy and the limited efficacy of current treatments, patients are turning to experimental therapies as alternatives to conventional TNBC treatment.
Innovations in Antibody-drug Conjugates (ADCs) are projected to create lucrative opportunities for key market players. Several pharmaceutical companies are focusing on launching new ADC candidates with fewer side effects and improved efficacy. An investigational ADC called Datopotamab Deruxtecan (Dato-DXd), for instance, has showcased favorable results in early trials, thereby becoming a significant future competitor.
Leading companies are further aiming to create combination therapies involving novel agents. According to preclinical research, TNBC response rates can be increased by pairing STAT3 inhibitors, BET inhibitors, or epigenetic modulators with existing therapies.
A leading trend is the rising number of clinical trials to discover new therapeutic targets. Conventional TNBC treatments mainly relied on chemotherapy. However, recent trials are focusing on new molecular targets such as the Notch signaling pathways, Wnt/β-catenin, and PI3K/AKT/mTOR. For instance, in the IPATunity130 trial, paclitaxel and the AKT inhibitor Ipatasertib were used to treat TNBC patients who had mutations in the PI3K/AKT pathway. Although the trial's findings were conflicting, they did support the possibility of using precision medicine to address the aggressive tumor biology of TNBC.
Based on the drug, the global market has been segmented into doxorubicin, cyclophosphamide, paclitaxel, docetaxel, and carboplatin/cisplatin. Out of these, the paclitaxel segment will likely generate a triple negative breast cancer treatment market share of around 57.8% in 2025, says Persistence Market Research.
It is attributed to the drug’s high effectiveness in combination therapies, mainly with new immune checkpoint inhibitors and targeted agents. The IMpassion130 trial, for example, exhibited that paclitaxel plus atezolizumab (Tecentriq) significantly improved progression-free survival (PFS) in patients with PD-L1-positive TNBC.
Doxorubicin is anticipated to witness steady growth through 2032 due to its synergistic effect when combined with chemotherapy agents such as taxanes. As it is widely accessible and reasonably priced, it continues to be the first-line treatment in settings with low resources.
Based on distribution channel, the market is bifurcated into hospital pharmacies and specialty cancer centers. Among these, the hospital pharmacies segment is projected to hold a share of 70.2% in 2025 and outpace specialty cancer centers. Hospital pharmacies are mainly preferred for TNBC treatment due to the need for close patient monitoring, stringent administration requirements, and specialized nature of therapies.
Hospital pharmacy's role in treating TNBC is further reinforced by the availability of new drugs and clinical trial accessibility. Hospital-led clinical trials, in which hospital pharmacies oversee drug delivery in accordance with stringent trial guidelines, offer several novel TNBC therapeutics.
Specialty cancer clinics, on the other hand, focus solely on oncology, enabling improved patient monitoring, easy access to novel drugs, and personalized treatment options. Leading centers such as MD Anderson Cancer Center and Memorial Sloan Kettering Cancer Center, for instance, have broadened their TNBC treatment programs to provide new options.
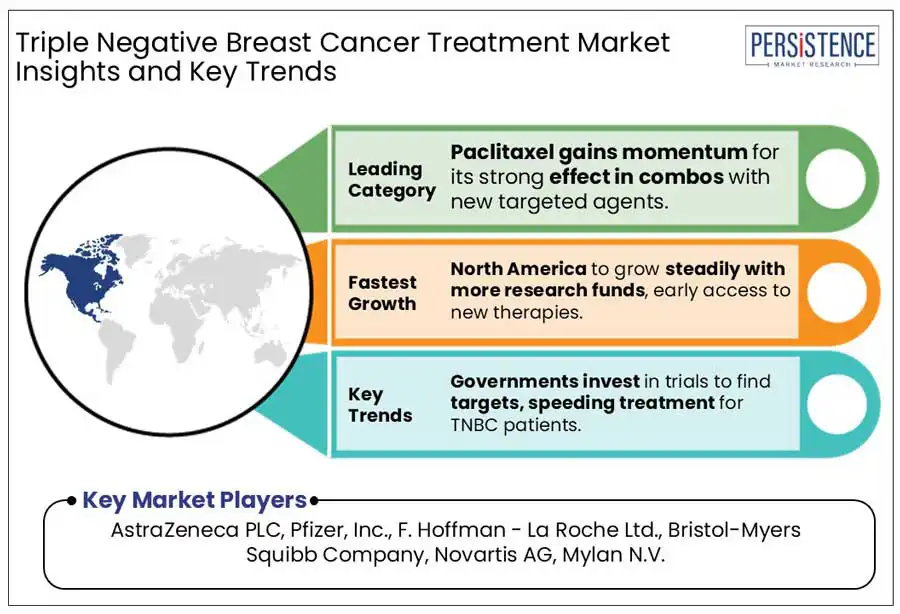
North America is estimated to account for a share of 39.6% in 2025. The regional growth is pushed by the U.S. triple negative breast cancer treatment market due to increasing research funding and early access to breakthrough therapies. The U.S. Food and Drug Administration (FDA) has approved several targeted therapies for TNBC, including Pembrolizumab (Keytruda), which have been significantly enhancing survival rates.
Both private and government agencies in the country are investing heavily in clinical trials, offering patients easy access to new treatments. The I-SPY 2 study, for instance, has been significant in evaluating novel TNBC treatments in a neoadjuvant context, which has sped up the approval of drugs such as Pembrolizumab and Atezolizumab.
In Asia Pacific, China is anticipated to show steady growth through 2032, backed by implementation of various cancer initiatives by the government and a booming biotechnology sector. Several TNBC therapies including Toripalimab and Camrelizumab have been sent for approval by the National Medical Products Administration (NMPA). These treatments are increasingly being utilized in combination with chemotherapy.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) recently approved Olaparib and Sacituzumab Govitecan, 2 important TNBC drugs, to ensure access to new targeted therapies. The country’s National Cancer Center (NCC) as well as other local research institutes are conducting in-depth studies on combination therapies, immune resistance mechanisms, and TNBC biomarkers. Daiichi Sankyo’s DS-1062, for example, is currently under clinical trial and is likely to become a key treatment option for TNBC in future.
Europe is projected to be spearheaded by Germany due to its extensive clinical trials and popularity of precision medicine. Research institutions such as Charité – Universitätsmedizin Berlin and the German Cancer Research Center (DKFZ) are conducting studies on molecular profiling and immunotherapy combinations of TNBC tumors. Easy availability of innovative diagnostic tools is another key factor contributing to market growth. Use of BRCA mutation testing and next-generation sequencing (NGS) helps oncologists create customized treatment plans.
The U.K. triple negative breast cancer treatment market is mainly supported by Cancer Research U.K. and the National Health Service (NHS). The NHS enables patients to obtain new treatments before full regulatory approval with its early-access schemes for novel therapies. The Cancer Drugs Fund (CDF) also provides promising treatments including immunotherapies and PARP inhibitors to eligible TNBC patients.
The global triple negative breast cancer treatment market is moderately competitive. Several healthcare firms and academic institutions are conducting research studies to come up with new treatment options. They are striving to find out novel options such as medicinal plants and FDA-approved drugs to raise the efficacy of treatment.
|
Report Attributes |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis Units |
Value: US$ Bn/Mn, Volume: As Applicable |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Drug Type
By Distribution Channel
By Region
To know more about delivery timeline for this report Contact Sales

The market is projected to be valued at US$ 1,130.3 Mn in 2025.
The market is estimated to rise at a CAGR of 5.8% through 2032.
AstraZeneca PLC, Pfizer, Inc., F. Hoffman - La Roche Ltd., Bristol-Myers Squibb Company, and Novartis AG are some leading providers.
The global market surged at a CAGR of 4.6% in the historical period.
Paclitaxel is considered the leading drug type.