ID: PMRREP5687| 199 Pages | 3 Oct 2025 | Format: PDF, Excel, PPT* | Healthcare

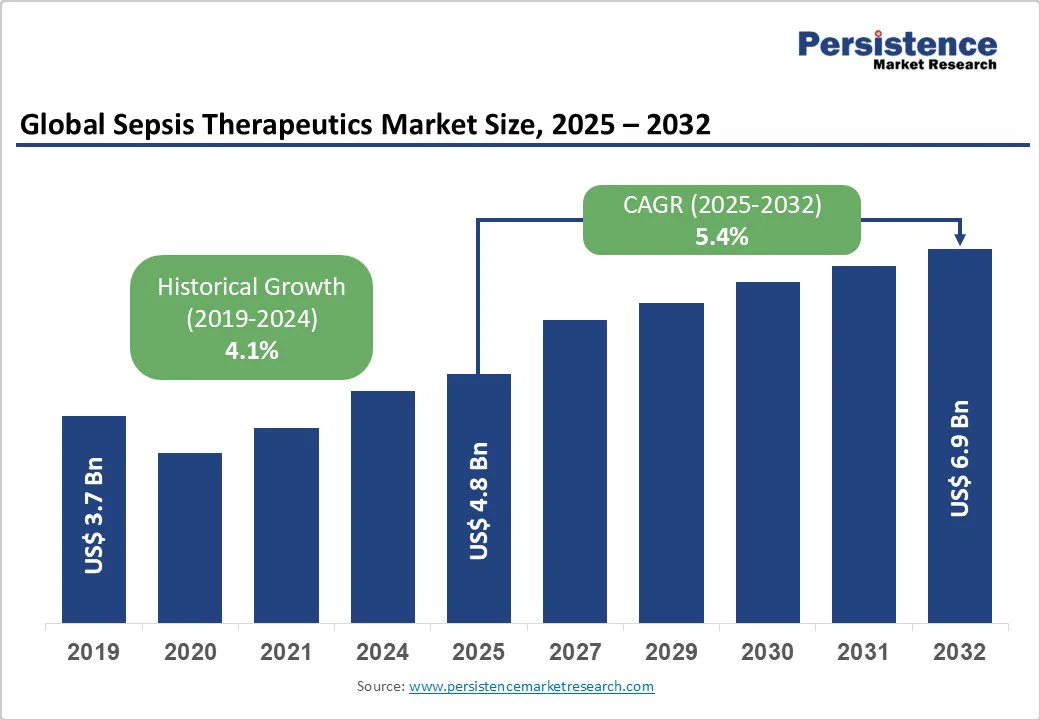
The global sepsis therapeutics market size is likely to value at US$4.8 Bn in 2025 and reach US$6.9 Bn by 2032, growing at a CAGR of 5.4% during the forecast period from 2025 to 2032.
The sepsis therapeutics market is witnessing robust growth, driven by increasing incidences of sepsis worldwide, advancements in diagnostic technologies, and rising demand for targeted antimicrobial treatments.
| Key Insights | Details |
|---|---|
| Sepsis Therapeutics Market Size (2025E) | US$ 4.8Bn |
| Market Value Forecast (2032F) | US$ 6.9Bn |
| Projected Growth (CAGR 2025 to 2032) | 5.4% |
| Historical Market Growth (CAGR 2019 to 2024) | 4.1% |
The global sepsis therapeutics market is experiencing accelerated expansion due to the rising prevalence of sepsis and hospital-acquired infections (HAIs). Sepsis, a severe response to infection leading to organ failure, affects millions annually, with the World Health Organization estimating 49 million cases and 11 million deaths in 2023 alone.
In the U.S., the Centers for Disease Control and Prevention reports over 1.7 million sepsis cases yearly, contributing to 270,000 deaths, underscoring the urgent need for effective therapeutics. This driver is amplified by the aging global population, where individuals over 65 are five times more susceptible, per the European Centre for Disease Prevention and Control.
Advanced diagnostics such as biomarker testing enable faster intervention, boosting demand for antibiotics such as cephalosporins. Pharmaceutical giants such as Pfizer Inc. reported a 15% sales uplift in sepsis drugs in 2024, driven by hospital protocols emphasizing early antibiotic administration.
Government initiatives, including the UK's Sepsis Six campaign, which reduced mortality in participating hospitals, further fuel adoption. As healthcare systems prioritize sepsis bundles-integrating rapid diagnostics, fluids, and antimicrobials-this driver ensures robust market momentum through 2032, particularly in intensive care units where timely therapeutics can improve survival rates by up to 50%, according to the Surviving Sepsis Campaign guidelines.
The sepsis therapeutics market encounters significant hurdles from elevated R&D expenses and the growing threat of antimicrobial resistance (AMR). Developing novel antibiotics requires substantial investments, with clinical trials for sepsis therapies facing high failure rates due to heterogeneous patient responses, as highlighted by a 2023 Tufts Center for the Study of Drug Development report. This financial burden deters smaller biotech firms, leading to market consolidation and limited innovation pipelines.
AMR further complicates efficacy, with the WHO projecting 10 million annual deaths by 2050 if unchecked, rendering traditional drugs such as aminoglycosides less effective against multidrug-resistant bacteria. In regions such as sub-Saharan Africa, resistance rates exceed 60% for common sepsis pathogens, per the Global Antibiotic Resistance Partnership, increasing treatment failures and healthcare costs.
Regulatory delays, such as the FDA's stringent post-approval surveillance for sepsis drugs, add to pricing pressures, making therapies unaffordable in low-income settings. These factors restrain market growth, particularly in cost-sensitive areas, where generic alternatives dominate but fail to address resistance, ultimately slowing the adoption of advanced therapeutics and necessitating diversified strategies to mitigate long-term impacts.
The sepsis therapeutics market is experiencing significant advancements driven by precision medicine, combination therapies, and technological innovations. In high-burden regions, particularly in Asia Pacific, the integration of telemedicine facilitates early intervention, enabling timely diagnosis and treatment.
For instance, China's National Health Commission is piloting AI-driven sepsis protocols, which integrate both oral and parenteral routes to enhance patient compliance and outcomes. Pharmaceutical companies are also contributing to this progress.
AstraZeneca is developing next-generation glycopeptides with improved pharmacokinetics, aligning with the FDA's Qualified Infectious Disease Product (QIDP) incentives that expedite approvals. These developments aim to address the growing concern of antimicrobial resistance and improve treatment efficacy.
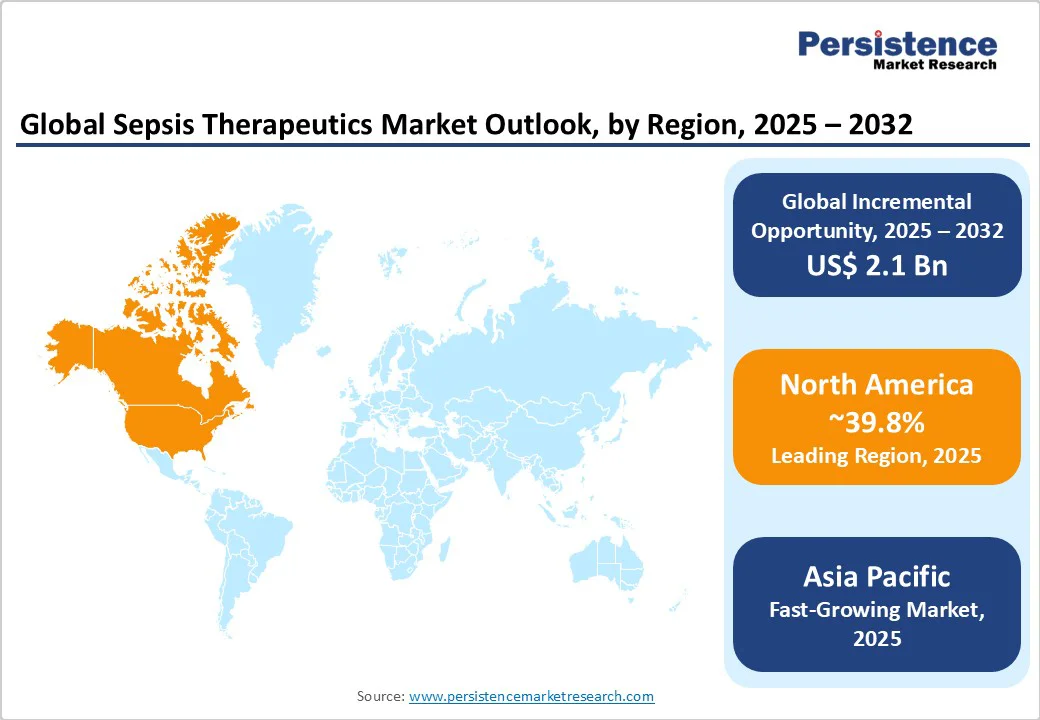
North America leads the global sepsis therapeutics market, accounting for a dominant 39.8% share. This leadership is primarily driven by the region’s advanced healthcare infrastructure, strong government and private sector investment in medical research, and a high incidence of sepsis.
In the United States alone, the Centers for Disease Control and Prevention (CDC) reports approximately 1.7 million sepsis cases annually, underscoring the urgent need for effective treatments. This high prevalence has spurred demand for innovative therapeutics and early diagnostic tools.
Furthermore, public health initiatives, such as those led by the Sepsis Alliance, play a critical role in raising awareness and promoting early intervention. These campaigns help improve patient outcomes by educating both healthcare professionals and the public about recognizing early signs of sepsis. Coupled with robust R&D pipelines and regulatory support in both the U.S. and Canada, North America remains at the forefront of driving advancements in sepsis management and treatment.
Asia Pacific is emerging as the fastest-growing region in the global sepsis therapeutics market, fueled by a combination of escalating infectious disease burdens, expanding healthcare infrastructure, and significant demographic pressures, particularly in densely populated countries such as China and India.
Rapid urbanization, aging populations, and increased antibiotic resistance have contributed to a higher incidence of sepsis across the region. In response, governments and the private sectors are investing heavily in upgrading healthcare facilities, improving diagnostic capabilities, and expanding access to critical care services.
Countries such as China and India are witnessing major healthcare reforms aimed at strengthening early detection and treatment protocols for sepsis. Moreover, growing awareness of the condition and its complications is driving demand for advanced therapeutics and medical technologies. International collaborations and clinical research initiatives are also increasing, positioning the Asia Pacific as a key growth engine in the global sepsis therapeutics landscape over the coming years.
Europe stands as the second fastest-growing region in the sepsis therapeutics market, driven by a combination of regulatory rigor, demographic trends, and strong research collaboration.
The European Union enforces strict healthcare regulations and patient safety standards, compelling hospitals and pharmaceutical companies to prioritize early sepsis detection and treatment. Additionally, Europe's aging population-particularly in countries such as Germany, Italy, and the UK has led to a higher susceptibility to sepsis, further increasing the demand for advanced therapeutic options.
Nations such as Germany and the United Kingdom are at the forefront of collaborative research and development efforts, leveraging public-private partnerships, academic research institutions, and biotech startups to innovate in sepsis care.
Funding from EU health programs and national health agencies supports clinical trials and the development of novel treatments. Coupled with rising awareness campaigns and digital health integration, Europe is poised to strengthen its role in the global effort to combat sepsis effectively.
The global sepsis therapeutics market is intensely competitive, characterized by strategic alliances, R&D investments, and a focus on novel drug deliveries. It operates as an oligopolistic landscape with major pharmaceutical powerhouses leveraging patents and global supply chains.
Regional variations exist, with U.S.-based firms dominating innovation while Asian players emphasize generics. Companies prioritize mergers, such as AstraZeneca's acquisitions, to expand portfolios in resistant pathogens, driven by regulatory incentives and unmet needs in critical care.
The Sepsis Therapeutics market is projected to reach US$4.8 Bn in 2025.
Escalating incidence of sepsis and hospital-acquired infections, alongside advancements in precision diagnostics, are the key market drivers.
The Sepsis Therapeutics market is poised to witness a CAGR of 5.4% from 2025 to 2032.
Advancements in precision medicine and combination therapies represent the key market opportunity.
Pfizer Inc., GSK plc, AstraZeneca, and Eli Lilly and Company are key market players.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2019 - 2024 |
| Forecast Period | 2025 - 2032 |
| Market Analysis | Value: US$ Bn/Mn, Volume: As Applicable |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
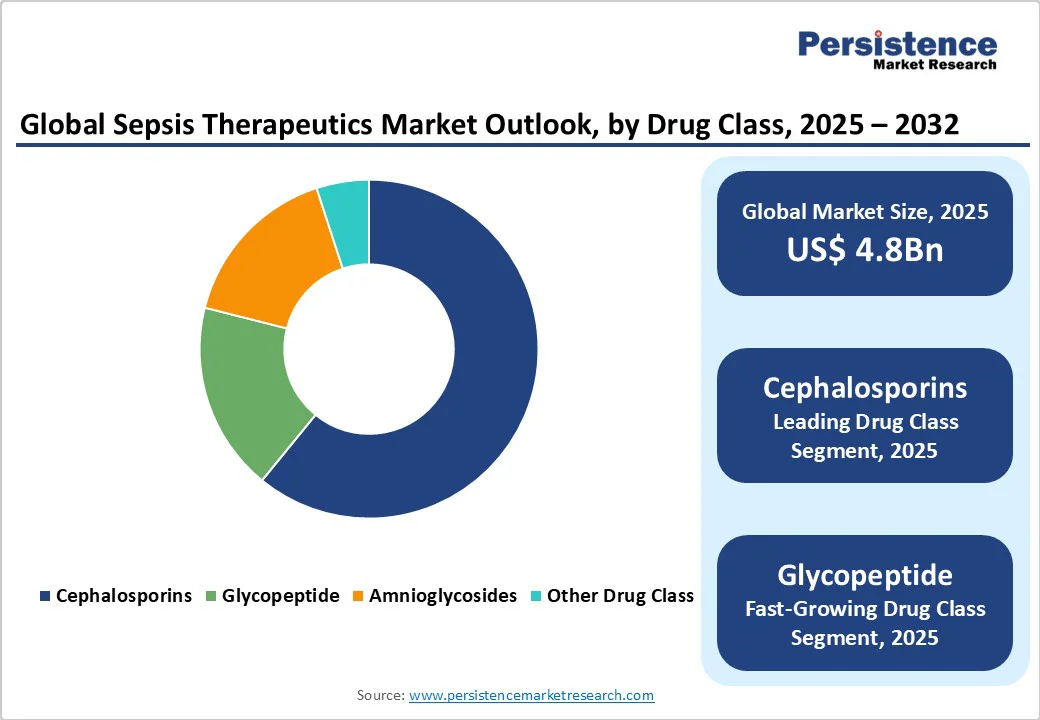
By Drug Class
By Route of Administration
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author