Comprehensive Snapshot of Renal Biomarker Research Report, Including Regional and Country Analysis in Brief.
Industry: Healthcare
Published Date: April-2025
Format: PPT*, PDF, EXCEL
Delivery Timelines: Contact Sales
Number of Pages: 200
Report ID: PMRREP35237
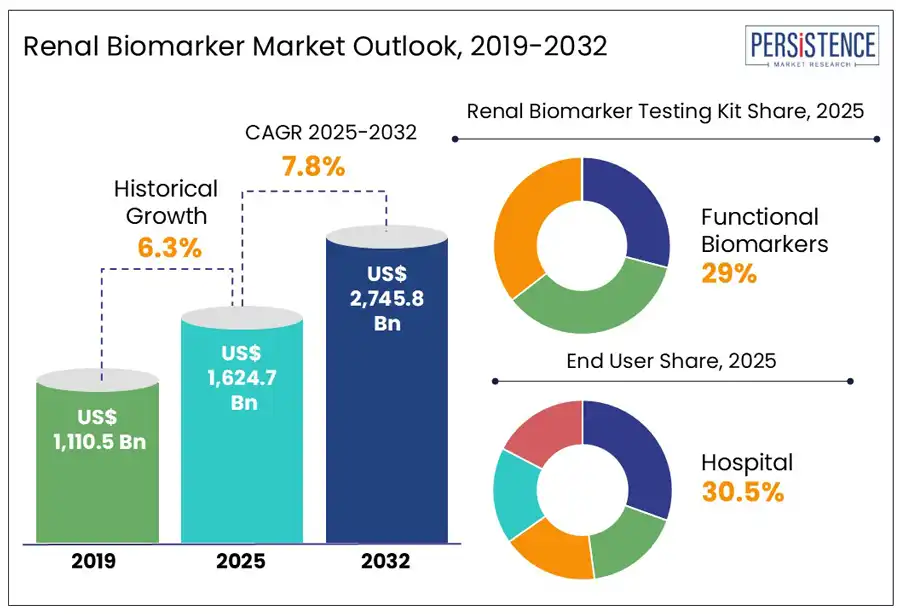
The global renal biomarker market size is anticipated to rise from US$ 1.6 Bn in 2025 to US$ 2.7 Bn by 2032. It is projected to witness a CAGR of 7.8% from 2025 to 2032. According to the Persistence Market Research report, the demand for renal biomarkers will likely skyrocket in the forecast period due to the global surging prevalence of various kidney disorders. 1 in 7 adults or around 14% people are estimated to have CKD (Chronic kidney Disorder) in the U.S. CKD is more common among people aged 65 or older (CDC, 2023).
Key Industry Highlights:
|
Global Market Attribute |
Key Insights |
|
Renal Biomarker Market Size (2025E) |
US$ 1.6 Bn |
|
Market Value Forecast (2032F) |
US$ 2.7 Bn |
|
Projected Growth (CAGR 2025 to 2032) |
7.8% |
|
Historical Market Growth (CAGR 2019 to 2024) |
6.3% |
The increasing prevalence of renal disorders such as glomerulonephritis, membranous nephropathy (MN), diabetic nephropathy, hypertensive nephropathy across the globe is set to create a high demand for renal biomarkers. In September 2024, Athenese Dx launched the ALTA ELISA Plate Reader, an advanced LED-based system designed to improve the accuracy and efficiency of ELISA assays, including those for renal biomarkers.
ELISA is a highly sensitive, specific, and cost-effective diagnostic tool that is preferred for identifying important biomarkers such as Neutrophil Gelatinase-Associated Lipocalin (NGAL), Kidney Injury Molecule-1 (KIM-1), and Cystatin C. Early detection of kidney diseases – CKD and AKI - improves treatment outcomes and increases the demand for ELISA-based diagnostic kits. Furthermore, advancements in automated ELISA systems, along with the rising prevalence of renal disorders worldwide, contribute to the growth of the market.
In low and middle-income countries (LMICs), inadequate healthcare infrastructure affects access to advanced renal biomarker testing. The scarcity of properly equipped laboratory facilities in certain areas makes conducting specialized diagnostics challenging. Moreover, the shortage of trained healthcare professionals, such as nephrologists, laboratory technicians, and pathologists, further leads to misdiagnosis or delayed identification of kidney diseases.
Limited funding and resources also constrain the availability of expensive biomarker testing. These obstacles result in unequal healthcare access, heightening the burden of CKD and deteriorating patient outcomes in settings with limited resources. CKD is a significant health issue in LMICs, affecting over 700 million people globally. Notably, 78% of individuals with CKD live in these nations, where health systems often struggle with limited resources and systemic inequities. (Elsevier Inc. 2024)
Biomarkers have significant potential to transform clinical research departments in the near future. Renal biomarkers provide real-time, objective data on patient health and treatment outcomes. With the technological advancements and regulatory frameworks, the renal biomarkers market is expected to integrate digital biomarkers into clinical research. These digital biomarker devices are expected to help uncover hidden insights, touchpoints, and connections.
The data collected through these devices is likely to offer strategic guidance for drug development in manufacturing companies involved in clinical research. Digital biomarker tools include smartwatches and sensor-embedded wearable devices that passively collect data. In June 2024, Researchers at IIT-BHU developed a nano-engineered paper microchip that detects kidney function biomarkers, specifically creatinine and albumin. The device uses a smartphone-based imaging system to measure creatinine levels, while a 3D-printed cascade is employed to detect albumin. An automated software called CretCheck interprets the results and provides immediate feedback on kidney health.
In the realm of product type, the functional biomarkers segment is expected to dominate the market, with a projected share of 29.0% by 2025. These testing kits are gaining traction due to their capacity to address various renal diagnoses. Common functional biomarkers, such as serum creatinine, cystatin C, and blood urea nitrogen (BUN), are widely utilized in clinical diagnostics for the early detection and monitoring of kidney diseases. These biomarkers offer essential insights into the glomerular filtration rate (GFR), establishing them as the gold standard for diagnosing CKD and AKI.
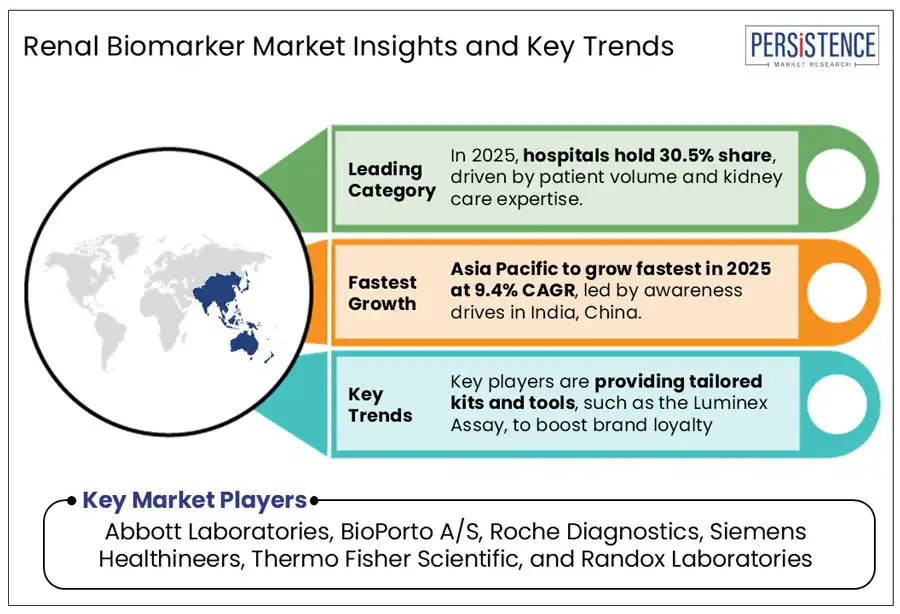
In 2025, the hospital segment is expected to hold a 30.5% share in the use of renal biomarkers. Healthcare facilities are the primary players in the renal biomarker market, largely due to their significant patient numbers for the diagnosis and treatment of kidney disorders. With the state-of-the-art diagnostic tools such as ELISA, LC-MS, and CLIA, they provide quick and precise identification of AKI, CKD, and various other renal issues.
The increasing global prevalence of kidney diseases, alongside the expanding use of personalized medicine, drives the need for accurate diagnostics. The extensive laboratories and dedicated nephrology departments in hospitals facilitate the integration of new biomarker tests and bolster research by using large patient datasets, which aids in the discovery and validation of innovative renal biomarkers.
North America is projected to account for 35.3% of the total market share by 2025. This growth is driven by the region's strong healthcare infrastructure, significant investments in research and development, and the presence of major market players that foster innovation. Emerging biomarker technologies, such as NGAL and KIM-1 are improving diagnostic accuracy. As the renal biomarker market in North America expands, it plays a vital role in bridging the gap between early diagnosis and effective management of kidney disease. As per the CDC, around 35.5 million Americans are affected by renal disease in the U.S. CKD is slightly more common in women (14%) than in men (12%).
Europe renal biomarker market is projected to capture 29.1% market share by 2025. The future of nephrology in Europe is transformed by the increasing importance of renal biomarkers for early disease detection, personalized treatment, and improved patient outcomes. These biomarkers facilitate accurate diagnosis, risk assessment, and real-time monitoring of CKD, AKI, and other renal disorders.
Advancements in omics technologies, artificial intelligence, and biomarker-driven clinical trials foster innovation in nephrology. In January 2023, Renalytix plc launched PRIME-CKD, a consortium funded by Horizon Europe. This initiative aims to validate and implement innovative biomarker-based tests to predict how patients with CKD will respond to currently available medications. Furthermore, the European healthcare system is progressively incorporating renal biomarkers into routine practice, thereby enhancing preventive care and therapeutic strategies.
Asia Pacific is estimated to account for a share of 22.3% in 2025. Rising awareness programs conducted by government and private agencies across India and China are projected to boost sales. In 2024, China launched the REVEAL-CKD study to address the underdiagnosis of early-stage chronic kidney disease (CKD). The initiative focuses on identifying characteristics of patients with undiagnosed stage 3 CKD, aiming to improve early detection and intervention strategies. By understanding these patients' profiles, the study seeks to enhance healthcare policies and outcomes for early-stage CKD in China.
The clinical industry in Asia Pacific is experiencing rapid growth, driven by innovations in diagnostic technologies and strong government healthcare initiatives. The rising incidence of chronic kidney disease (CKD) and an increasing awareness of early detection has boosted the demand for advanced biomarkers. Additionally, supportive regulatory frameworks and research funding are further accelerating market expansion.
The global renal biomarker market is highly competitive with leading companies dominating the landscape through their integrated business operations. Key players are focusing on launching new products with unique features, strengthening their distribution networks, and expanding into new geographical areas.
Leading players in the diagnostics industry, such as Abbott Laboratories, BioPorto A/S, Roche Diagnostics, Siemens Healthineers, Thermo Fisher Scientific, and Randox Laboratories are investing in advanced diagnostic techniques to improve the accuracy of biomarker detection.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis Units |
Value: US$ Bn Volume: Unit |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Product
By Diagnostic Technique
By End-use
By Region
To know more about delivery timeline for this report Contact Sales

The market is set to reach US$ 1.6 Bn in 2025.
Functional biomarkers segment is projected to dominate in the forecast period globally.
The industry is estimated to rise at a CAGR of 7.8% through 2032.
North America is projected to hold the largest share in 2025.
Abbott Laboratories, BioPorto A/S, Roche Diagnostics, Siemens Healthineers, Thermo Fisher Scientific, and Randox Laboratories Incorporated are the leading players.