Industry: Healthcare
Published Date: January-2025
Format: PPT*, PDF, EXCEL
Delivery Timelines: Contact Sales
Number of Pages: 185
Report ID: PMRREP31310
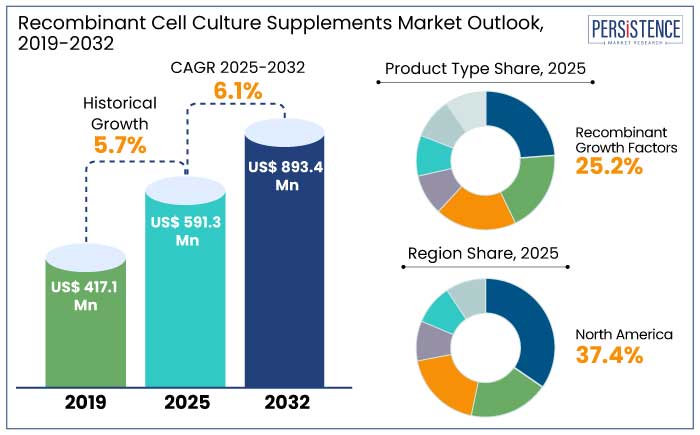
The global recombinant cell culture supplements market is predicted to reach a size of US$ 591.3 Mn by 2025. It is anticipated to experience a CAGR of 6.1% during the forecast period to attain a value of US$ 893.4 Mn by 2032.
The shift toward serum-free and animal-free cell culture media, owing to concerns regarding contamination, ethical consideration, and regulatory restrictions is a prominent trend fostering growth. Recombinant supplements are increasingly preferred for their high purity, consistent quality, and ability to minimize contamination risks.
Growing global burden of chronic diseases like cancer, diabetes, and autoimmune disorders has resulted in an increased demand for biopharmaceuticals. Recombinant supplements are essential in the production of monoclonal antibodies, vaccines, and other biologics.
Key Highlights of the Industry
|
Market Attributes |
Key Insights |
|
Recombinant Cell Culture Supplements Market Size (2025E) |
US$ 591.3 Mn |
|
Projected Market Value (2032F) |
US$ 893.4 Mn |
|
Global Market Growth Rate (CAGR 2025 to 2032) |
6.1% |
|
Historical Market Growth Rate (CAGR 2019 to 2023) |
5.7% |
North America recombinant cell culture supplements market is estimated to hold a share of 37.4% in 2025. The region, particularly the U.S., is home to some of the largest biopharmaceutical companies, including Pfizer, Amgen, and Johnson & Johnson. These companies are prominent consumers of recombinant cell culture supplements in their production processes. For example,
North America is witnessing a rapid demand for biologics like monoclonal antibodies and vaccines. Recombinant cell culture supplements are essential in the production of these biologics.
FDA and other regulatory bodies in the region have stringent guidelines that promote the use of serum-free and animal-free cell culture media. Recombinant supplements align with these guidelines as they are safer and ethically sourced.
Recombinant growth factors are anticipated to hold a share of 25.2% in 2025. These products are vital for stimulating cell proliferation and maintaining cell viability in culture systems.
Recombinant growth factors ensure high-quality cell cultures which is particularly crucial for applications in biopharmaceutical manufacturing, regenerative medicine, and vaccine development. Companies are investing in innovative recombinant growth factor products to enhance cell culture efficiency. For instance,
Recombinant products also enable large-scale production with consistent quality, thereby addressing the needs of the expanding biopharma and cell therapy industries. Advances in recombinant technology have minimized the production costs of growth factors, making them more accessible to biopharmaceutical manufacturers.
Stem cell therapy is anticipated to hold a share of 30.6% in 2025. It is at the forefront of regenerative medicine as it addresses the repair and replacement of damaged tissues and organs.
Government authorities and private investors are heavily funding stem cell research and therapy development. Global funding for stem cell research surpassed US$ 10 Bn in 2023, their bey boosting the demand for high-quality recombinant cell culture supplements.
Stem cell therapy is a cornerstone of personalized medicine, offering tailored treatments for individual patients. Recombinant supplements provide the precise growth factors and cytokines needed to develop patient-specific therapies.
Potential growth in the global recombinant cell culture supplements industry is predicted to be driven by growing focus on sustainable and ethically sourced products, thereby driving adoption, especially in regions having stringent regulatory frameworks. The assessment period is likely to witness expansion in applications of stem cell therapy in regenerative medicine and personalized medicine. Emerging markets are predicted to witness an increased adoption of recombinant supplements.
The recombinant cell culture supplements market growth was steady at a CAGR of 5.7% during the historical period. Growth during the period was primarily driven by the increased demand for biologics, like monoclonal antibodies (mAbs) and vaccines, which are dependent on recombinant supplements for scalable and high-quality production.
Adoption of recombinant supplements in stem cell research and therapy development witnessed rapid growth. The COVID-19 pandemic accelerated vaccine development, with recombinant cell culture supplements playing a crucial role in scaling up vaccine production.
The forecast period is estimated to witness advancements in recombinant technologies like site-specific mutagenesis and protein engineering. These developments are likely to introduce high-performance supplements with enhanced efficacy.
Transition to Serum-free Media
Conventional serum-based media, particularly those derived from fetal bovine serum (FBS), carry risks of contamination with viruses, mycoplasma, and prions. These risks are especially critical in the production of biologics where contamination can lead to batch failures and regulatory complications. Serum-free media, supplemented with recombinant growth factors, cytokines, and hormones, provide a consistent and contamination-free alternative.
Use of FBS and other animal-derived supplements faces increased scrutiny owing to ethical concerns regarding animal welfare. Regulatory agencies like the FDA and EMA have been advocating for the use of animal-free products in biopharmaceutical manufacturing to ensure ethical and consistent production practices. For instance,
Serum-free media ensures consistent composition, eliminating batch-to-batch variability associated with serum-based media. Recombinant supplements enhance the scalability of cell culture processes by providing reliable performance across different production scales. Serum-free formulations can be tailored for specific cell types, enhancing growth rates and productivity.
Expansion of Vaccine Development
Growing prevalence of infectious diseases, like influenza, hepatitis, and threats like COVID-19, has increased the demand for vaccines.
Modern vaccines like mRNA-based and viral vector vaccines require sophisticated cell culture systems for development and production. Recombinant cell culture supplements are critical for supporting the large-scale production of these advanced vaccine platforms. For instance,
Contamination Risks and Quality Control Issues
Cell culture systems used to produce recombinant proteins, growth factors, and other supplements are highly sensitive to environmental conditions, and contamination can have serious consequences. Risk of contamination is multifaceted and can arise from various sources, including microorganisms, chemical impurities, and human error.
One of the most common types of contamination in recombinant cell culture systems is microbial contamination. Even small amounts of microbial contamination can lead to significant product loss and affect the integrity of cell culture systems.
Emerging Applications in Tissue Engineering
Tissue engineering addresses the critical shortage of donor organs and the growing demand for regenerative treatments. Regenerative medicine is heavily reliant on advanced cell culture techniques including recombinant supplements to develop engineered tissues and organs.
Tissue engineering requires precise and contamination-free environments for cell growth and tissue formation. This makes recombinant growth factors, cytokines, and proteins indispensable. Recombinant supplements ensure consistent quality while decreasing variability compared to traditional serum-based media, which is critical for generating functional tissue constructs.
Developments in Recombinant Technology
Innovations in technology have streamlined the production of recombinant cell culture supplements, enabling higher yield, lower cost, and decreased batch-to-batch variability. For instance,
New expression systems, like mammalian cells, yeast, and insect cells, have been developed to optimize the production of recombinant supplements with enhanced functionality and stability. Innovations in yeast-based systems have significantly decreased production costs, making recombinant supplements more accessible to academic and research institutions.
Integration of automation and bioprocess optimization in recombinant technology has enabled large-scale production of cell culture supplements with consistent quality.
Companies in the recombinant cell culture supplements market are innovating their product offerings to stay relevant. They are progressively investing in research and development activities to develop new and improved recombinant cell culture supplements with enhanced performance, purity, and specificity.
Businesses are focusing on expanding their product portfolios to cater to a wide range of application. They are introducing specialize supplements to support the culture of stem cells and other cell types used in these therapies.
Manufacturers are launching new supplements that facilitate tissue regeneration an scaffold-based growth. They are focused on providing cell culture supplements that support the growth of mammalian cells used in large-scale biologics production.
Recent Industry Developments
|
Attributes |
Detail |
|
Forecast Period |
2025 to 2032 |
|
Historical Data Available for |
2019 to 2023 |
|
Market Analysis |
US$ Million for Value |
|
Key Regions Covered |
|
|
Key Market Segments Covered |
|
|
Key Companies Profiled in the Report |
|
|
Report Coverage |
|
|
Customization and Pricing |
Available upon request |
By Product
By Source
By Application
By End User
By Region
To know more about delivery timeline for this report Contact Sales

The market is anticipated to reach a value of US$ 893.4 Mn by 2032.
In India, 12 recombinant therapeutics are presently being marketed.
North America is anticipated to emerge as the leading region with a share of 37.4% in 2025.
Prominent players in the market include Merck KGaA, Thermo Fisher Scientific Inc., and GE Healthcare.
The market is predicted to witness a CAGR of 6.1% throughout the forecast period.