Industry: Healthcare
Published Date: March-2025
Format: PPT*, PDF, EXCEL
Delivery Timelines: Contact Sales
Number of Pages: 187
Report ID: PMRREP32880
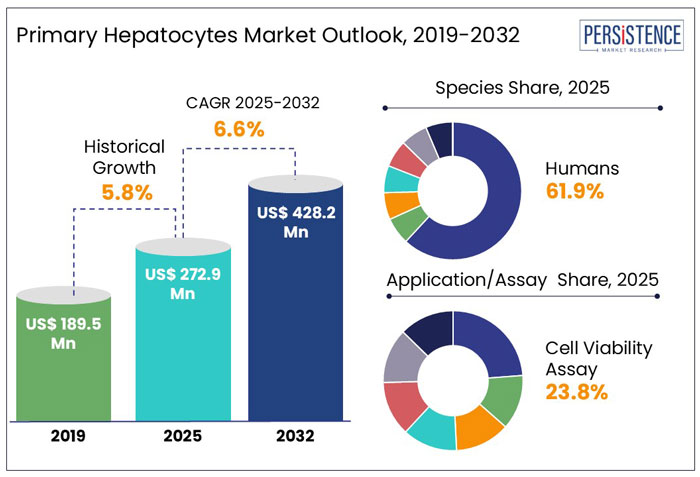
According to Persistence Market Research, the global primary hepatocytes market is projected to witness a CAGR of 6.6% during the forecast period from 2025 to 2032. It is anticipated to increase from US$ 257.2 Mn recorded in 2024 to a staggering US$ 428.2 Mn by 2032.
The global primary hepatocytes market is categorized into North America, Europe, East Asia, South Asia & Oceania and Middle East & Africa. The key competitive global market players such as Thermo Fisher Scientific Inc., Merck KGaA, Lonza, Takara Bio Inc., American Type Culture Collection (ATCC), Corning Inc.
The Primary Hepatocytes Market is experiencing growth due to the increasing demand for hepatocytes in drug discovery and toxicology studies. As pharmaceutical companies and research institutions prioritize efficient drug screening processes, the use of primary hepatocytes for in vitro studies offers a reliable model for human liver functions.
Rising incidences of liver diseases, including hepatitis and non-alcoholic fatty liver disease (NAFLD), are further driving the need for hepatocyte research. Additionally, advancements in 3D cell culture technology and growing investments in personalized medicine bolster the market's expansion by enhancing hepatocyte functionality and application in regenerative medicine.
Key Highlights of the Market
|
Market Attributes |
Key Insights |
|
Global Primary Hepatocytes Market Size (2025E) |
US$ 272.9 Mn |
|
Projected Market Value (2032F) |
US$ 428.2 Mn |
|
Global Market Growth Rate (CAGR 2025 to 2032) |
6.6% |
|
Historical Market Growth Rate (CAGR 2019 to 2024) |
5.8% |
Rise in Frequency of Liver Diseases Across the World Presented Challenges in Market
Improvements in cell isolation and culture methods propelled the global primary hepatocytes market's CAGR of 5.8% between 2019 and 2024. The growing use of hepatocyte-based models in toxicity testing and drug development, especially in the pharmaceutical and academic research sectors, is expanding the market.
Applications in organ-on-chip systems, regenerative treatments, and personalized medicine are anticipated to drive market growth. Hepatocytes will be more relevant for researching complicated liver disorders and functions owing to advancements in gene editing and co-culture models.
Shift Away from Animal Testing Drives Growth in Primary Hepatocytes Market
Animal testing has long been a cornerstone of preclinical research, but the transition toward in vitro organ-on-chip systems is revolutionizing liver toxicity testing. Studies have shown that these advanced models can reduce animal usage by 30% to 50% in pharmaceutical studies, offering a more ethical and efficient approach.
Meanwhile, the U.S. FDA's Modernization Act 2.0 now permits human-relevant in vitro models to replace animal studies in drug approval processes. These regulatory advancements are driving investment in alternative liver toxicity testing solutions.
A recent study, conducted with male C57BL/6J and db/db mice and approved by the Institutional Animal Care and Use Committee of Peking University Health Science Center, underscores the ongoing shift away from traditional animal testing.
This transition presents a lucrative opportunity for the primary hepatocytes market, which is projected to report a CAGR of 6.6%, reaching US$ 428.2 Mn by 2033.
Growth Drivers
Rising Incidence of Liver Diseases Driving Demand for Advanced Hepatocyte Models
The increase in liver illnesses such as cirrhosis, cancer, and nonalcoholic fatty liver disease is driving the demand for primary hepatocytes.
The emphasis of drug developers on liver-related medicines, developments in regenerative medicine, and bioengineered liver models are anticipated to drive a large growth in the global primary hepatocyte market.
With increasing research funding, the market is poised for sustained growth in the projected period.
Ethical and Regulatory Hurdles in Human Liver Tissue Sourcing
The ethical and regulatory scrutiny of sourcing human liver tissue for research and therapeutic applications is a significant challenge for researchers and biotech firms. Regulatory bodies enforce informed consent protocols, but regional regulations create logistical roadblocks, delaying access to viable tissue and stifling drug discovery and regenerative medicine advancements.
The global demand for human tissues for research purposes was valued at US$ 5.8 Bn in 2023, with liver tissues constituting a portion of hepatocyte-based drug metabolism and toxicity studies. Yet, concerns over exploitation, black-market trade, and public mistrust hinder broader acceptance.
In response, induced pluripotent stem cells (iPSCs) are emerging as an ethical and scalable alternative, with the stem cell therapy market projected to reach US$ 26.2 Bn by 2030. Establishing standardized ethical frameworks and investing in lab-grown hepatocytes could ensure a sustainable, compliant, and scientifically robust supply chain.
Expanding Role of Primary Hepatocytes in Liver Research and Therapy
Primary hepatocytes are essential for protein synthesis, detoxification, and metabolism, which makes them invaluable in the study of liver illness and regenerative medicine. Researchers are looking at hepatocyte-based cell therapies as potential liver transplant substitutes since chronic liver illnesses such as cirrhosis, hepatitis, and NAFLD are becoming more common and impact over 100 million people worldwide. These treatments provide organ failure patients with a more accessible and less intrusive option.
The demand for primary hepatocytes is expanding because of advancements in 3D cell culture technologies, especially for the development of cancer treatments because these cells can replicate physiological conditions more accurately.
Pharmaceutical companies are incorporating primary hepatocytes into drug screening models, improving toxicology and metabolism research, and leading to safer liver disease treatments.
Species Insights
Human Hepatocytes Lead Market as Demand for Accurate Liver Models Grows
In 2025, the human hepatocyte segment dominates the global hepatocyte market with a 61.9% share, driven by increased drug testing, liver disease research, and advancements in in vitro models. Human hepatocytes offer high physiological relevance, making them essential for pharmacokinetics, toxicity studies, and personalized medicine.
The rising prevalence of liver diseases, including NAFLD and hepatitis, is fueling demand. Although rats which account for 10.3%, along with other species like dogs and monkeys’ hepatocytes are still used in preclinical drug research, the use of these sources is declining due to the need for non-animal substitutes. For instance,
Similarly, Lonza Group expanded its primary hepatocyte portfolio in January 2024, offering high-purity human and animal hepatocytes to enhance predictive liver models for pharmaceutical applications.
Application/Assay Insights
Innovations in Cell Viability Assays Drive Growth in Market with Reduced Animal Model Reliance
The cell viability assay segment is projected to account for 23.8% of the global primary hepatocytes market in 2025, fueled by its critical role in drug toxicity and efficacy studies. These assays help evaluate liver cell health, metabolism, and drug-induced toxicity, making them essential for pharmaceutical research and development and precision medicine.
The demand for high-throughput and automated cell viability assays is increasing as companies adopt non-animal testing models. For instance,
Likewise, Thermo Fisher Scientific launched a new viability assay kit in January 2024, improving predictive toxicology applications.
With global pharmaceutical research and development spending projected to reach US$ 254 Bn by 2026, regulatory bodies like the FDA and EMA are encouraging safer, in vitro testing methods, accelerating market expansion.
Increase in Investments for Research and Development Programs Presents Prospects in North America
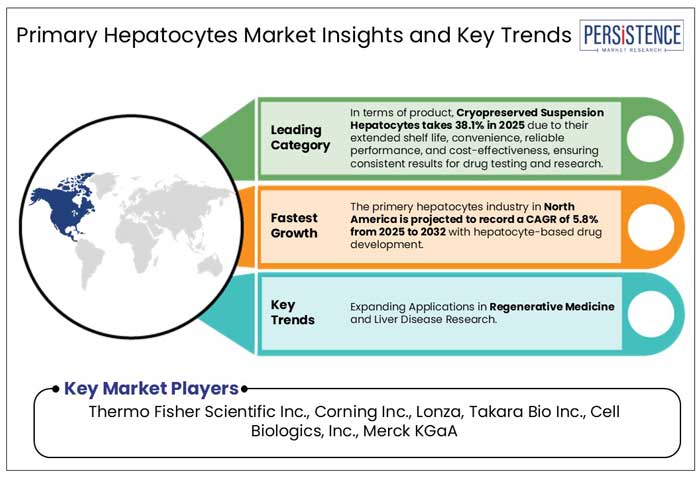
North America is set to hold a market share of 33.3% of the primary hepatocytes market in 2025, driven by strong research and development investments, advanced healthcare infrastructure, and pharmaceutical innovations. With North America at the forefront of hepatocyte-based drug development, the market is expected to witness steady growth with a projected CAGR of 5.8% through 2032.
The region’s leading biotechnology and pharmaceutical companies, such as Pfizer, Thermo Fisher Scientific, and Lonza, are investing heavily in cell culture technologies to advance drug discovery and personalized medicine. For instance,
Additionally, studies conducted by several government organizations emphasized the importance of hepatocyte models in understanding liver metabolism and toxicity, reinforcing their significance in toxicology and pharmacokinetics studies. Such as the U.S. FDA’s push for alternative testing models has increased the adoption of in vitro liver models, while government funding for cell-based research continues to accelerate market expansion.
Europe's Biotech and Pharma Sector Paves the Way for Investment in Primary Hepatocytes Research
In 2025, Europe is estimated to gain prominence in the primary hepatocytes market, holding 29.7%, with Germany, the U.K., and France leading in research infrastructure and innovation. Biotechnology and pharmaceutical industries are using primary hepatocytes for drug metabolism, enzyme activity studies, and toxicity screening.
The European Medicines Agency (EMA) has introduced guidelines to reduce animal testing and promote in vitro liver models, boosting market adoption.
With Europe’s regulatory push and biotech sector expansion, the demand for primary hepatocytes in research and industrial applications is expected to grow significantly through 2032.
East Asia Gains Prevalence with Adoption of Modern Technologies in Hepatocyte Differentiation
East Asia has solidified its position as a global leader in pharmaceutical research, with China, Japan, and South Korea driving demand for primary hepatocytes in drug discovery and liver disease research. In 2025, the market in East Asia is predicted to account for 19.1%.
Advances in cell isolation techniques have significantly improved efficiency and cell yield, ensuring a stable supply for researchers. For instance,
The demand for primary hepatocytes in regenerative medicine and personalized drug development is set to rise, particularly as research institutions in East Asia integrate advanced cell culture technologies for preclinical studies and liver disease modeling.
The global primary hepatocytes market is highly competitive, with numerous players focusing on innovation and quality to gain a market edge. Key companies include Thermo Fisher Scientific, Lonza Group, and BioIVT, which dominate due to their extensive product portfolios and advanced hepatocyte isolation technologies.
Smaller players and startups are also entering the market, leveraging niche capabilities like customized hepatocyte solutions and advanced cryopreservation techniques. Partnerships between biotech firms and research institutions drive innovation, while regional players are strengthening their positions through localized manufacturing and distribution networks.
The competition is fueled by growing demand for human-derived hepatocytes, pushing companies to enhance supply chain reliability, expand R&D efforts, and explore emerging markets.
Recent Industry Developments
|
Attributes |
Details |
|
Forecast Period |
2025 to 2032 |
|
Historical Data Available for |
2019 to 2024 |
|
Market Analysis |
US$ Million for Value |
|
Key Countries Covered |
|
|
Key Market Segments Covered |
|
|
Key Companies Profiled in the Report |
|
|
Report Coverage |
|
|
Customization & Pricing |
Available upon request |
By Product
By Species
By Application/Assay
By End User
By Region
To know more about delivery timeline for this report Contact Sales

The global market is expected to reach US$ 272.9 Mn in 2025.
Yes, the market is set to reach US$ 428.2 Mn by 2032.
North America is estimated to witness a high market share in 2025.
Thermo Fisher Scientific Inc., Merck KGaA are considered the leading players.
The three zones of the liver sinusoid and oxygen gradient are periportal, intermediate, and perivenous, pericentral, or centrilobular.