Industry: Healthcare
Published Date: September-2024
Format: PPT*, PDF, EXCEL
Delivery Timelines: Contact Sales
Number of Pages: 177
Report ID: PMRREP34757
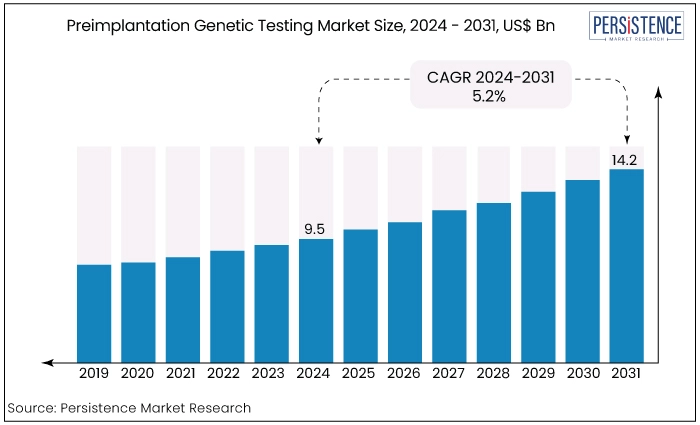
The preimplantation genetic testing market is estimated to reach a valuation of US$1.48 Bn by 2031 from the value US$0.68 Bn recorded in 2024. The market is expected to record a CAGR of 9%, during the forecast period between 2024 and 2031.
Key Highlights of the Market
|
Market Attributes |
Key Insights |
|
Preimplantation Genetic Testing Market Size (2024) |
US$0.68 bn |
|
Preimplantation Genetic Testing Market Size (2032) |
US$1.48 bn |
|
Forecast Growth Rate (CAGR 2024 to 2032) |
9% |
|
Historical Growth Rate (CAGR 2019 to 2023) |
7.8% |
|
Region |
Market Share in 2024 |
|
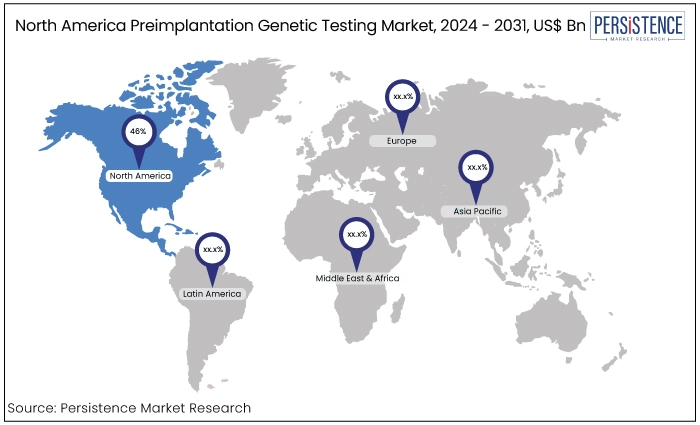
North America |
46% |
North America continues to lead the market in terms of revenue in 2024 accounting for 46% market share. It establishes itself as the market leader. The increase in healthcare expenditure and population size is the reason for the region’s dominance.
According to the data from the United States Department of Health and Human Services, there are around 500 fertility clinics that provide assisted reproductive technology (ART) options. States such as Massachusetts, Columbia, and New Jersey have the most significant incidence of newborns resulting from assisted reproductive technologies.
Increasing recognition of the significance of healthy embryos in nations with lower socioeconomic conditions is expected to create a lucrative prospect for preimplantation genetic diagnostics.
|
Category |
Market Share in 2024 |
|
Procedure - Genetic Diagnosis |
76% |
Based on procedure, the preimplantation genetic testing market is segmented into genetic diagnosis and genetic screening. Among these, the genetic diagnosis type dominates the market with market share of 76%.
The segment's rise is primarily driven by the increasing awareness among healthcare professionals and consumers regarding genetic testing for specific gene variants. The increasing prevalence of single gene illnesses and translocation instances along with the successful use of Next-Generation Sequencing (NGS) technology is driving this expansion.
PGD services provide the analysis of embryos to detect particular gene mutations, hence decreasing the occurrence of these diseases in new-borns.
|
Category |
Market Share in 2024 |
|
Product - Reagents |
51% |
Based on product, the preimplantation genetic testing market is segmented into reagents and instruments. Among these, the reagents segment dominates the market accounting for 51% market share. The increasing utilization of preimplantation genetic testing (PGT) in the In-Vitro Fertilization (IVF) procedure drives the need for reagents and consumables.
Every year, there are over 3.0 million IVF rounds conducted globally leading to more than 700,000 successful deliveries through IVF. Furthermore, the increasing adoption of sophisticated testing technologies such as next-generation sequencing, polymerase chain reaction, and fluorescence in-situ hybridization is anticipated to propel the expansion of this segment.
Preimplantation genetic testing also known as preimplantation genetic diagnosis involves screening embryos developed through in-vitro fertilization for genetic defects before uterine implantation.
Preimplantation genetic testing allows medical experts to detect genetic abnormalities in an embryo and intentionally implant only healthy embryos into the uterus. This testing enhances the mother's probability of giving birth to a child with a consistent genetic composition. Preimplantation genetic testing allows individuals to minimize the likelihood of transmitting hereditary disorders common in their families to their children.
The preimplantation procedures encompass a range of operations, including retrieving eggs from either the mother or an egg donor, which are subsequently fertilized in a laboratory setting. Following fertilization, the eggs undergo screening techniques to detect different genetic disorders. Viable embryos can be cryopreserved for future use, while nonviable embryos are disposed of.
The purpose of implanting healthy embryos is to achieve pregnancy successfully. Preimplantation genetic testing also has the additional function of allowing for choosing a child's gender. However, the current utilization of technology is accompanied by numerous ethical problems.
Individuals with sex-linked genetic conditions, single gene donors, individuals with chromosomal abnormalities, and older women seeking pregnancy are increasingly opting for preimplantation genetic testing as a therapeutic option for infertility.
The preimplantation genetic testing market share experienced robust growth leading up to 2023, driven by advancements in genetic technology and increasing awareness of genetic disorders. Key factors fueled the market growth include rising infertility rates, advances in in-vitro fertilization (IVF) techniques, and growing demand for healthy pregnancies and babies.
Technological advancements such as next-generation sequencing (NGS) and improved embryo biopsy techniques enhanced the accuracy and accessibility of PGT, further propelling market growth. North America and Europe dominated the market due to high healthcare expenditure, sophisticated healthcare infrastructure, and increased acceptance of genetic testing.
Post-2024, the PGT market is anticipated to continue its upward trajectory with an expected CAGR of 9%. Innovations in genetic screening technologies such as CRISPR and expanded carrier screening are likely to drive market expansion.
Personalized medicine trends and the push for precision health are expected to boost demand for PGT further. However, ethical concerns and regulatory challenges could pose constraints. Nonetheless, the global push for better reproductive health outcomes and the continued evolution of genetic technologies position the PGT market for sustained growth.
Advances in Genetic Technology
The rapid advancement in genetic technology is a significant driver for the preimplantation genetic testing market. Techniques such as next-generation sequencing (NGS) and CRISPR have revolutionized the field allowing for more accurate, efficient, and comprehensive genetic screening.
NGS in particular enables the simultaneous analysis of multiple genes reducing the time and cost associated with genetic testing. These advancements improve the precision of detecting genetic anomalies in embryos and enhance the overall success rates of IVF procedures.
Continuous development of non-invasive testing methods makes PGT more accessible and acceptable to prospective parents. As these technologies become more refined and widely adopted, the demand for PGT is expected to grow driven by the desire for healthier pregnancies and the prevention of genetic disorders.
Rising Infertility Rates and Delayed Childbearing
The increasing incidence of infertility and the trend toward delayed childbearing are key factors driving the preimplantation genetic testing market growth. Modern lifestyle factors such as stress, poor diet, and environmental pollutants contribute to rising infertility rates globally.
More couples choose to have children later in life due to career and personal preferences. Advanced maternal age is associated with a high risk of chromosomal abnormalities in embryos making PGT an essential tool for older prospective parents.
By enabling the selection of genetically healthy embryos, PGT improves the chances of successful pregnancies and reduces the risk of genetic disorders. The growing awareness and acceptance of assisted reproductive technologies (ART) further support the demand for PGT as couples seek to ensure the health and viability of their pregnancies.
Growing Acceptance and Awareness
Increasing awareness and acceptance of genetic testing are significant drivers of the PGT market. As more people become informed about the benefits of genetic screening, the demand for PGT rises.
Educational campaigns by healthcare providers, advancements in genetic counselling, and increased media coverage of genetic disorders contribute to this growing awareness. Furthermore, the stigma surrounding genetic testing and assisted reproductive technologies is gradually diminishing leading to broader acceptance.
Couples are more willing to invest in PGT to ensure healthy pregnancies and reduce the risk of passing on genetic disorders. Insurance companies are also beginning to recognize the long-term benefits of covering PGT further driving market growth.
High Costs and Limited Accessibility
One of the primary restraints for the preimplantation genetic testing market is the high cost of the procedure. The advanced technologies and specialized skills required for PGT make it an expensive option, which can be prohibitive for many couples. This financial barrier is especially significant in developing countries with limited healthcare infrastructure and insurance coverage for such procedures.
The accessibility of PGT remains restricted to a relatively small segment of the population primarily those in higher income brackets or regions with robust healthcare systems. Additionally, the lack of widespread insurance coverage for PGT further exacerbates this issue limiting the market’s growth potential by restricting access to those who can afford out-of-pocket expenses.
Ethical and Legal Concerns
Ethical and legal concerns pose another significant restraint on the growth of the PGT market. The process of selecting embryos based on genetic screening raises complex ethical questions about eugenics, designer babies, and the potential for genetic discrimination. These concerns can lead to public apprehension and opposition, impacting the overall acceptance and adoption of PGT.
Regulatory environments vary significantly across different countries with some imposing strict regulations or outright bans on specific aspects of genetic testing. Navigating these legal frameworks can be challenging for companies operating in the PGT market potentially limiting their ability to expand and innovate.
The ongoing ethical debates and regulatory uncertainties create a cautious market landscape hindering the full realization of PGT's potential benefits.
Integration of Personalized Medicine and Precision Health
The integration of personalized medicine and precision health presents a significant opportunity for the preimplantation genetic testing (PGT) market. Personalized medicine aims to tailor medical treatment to the individual characteristics of each patient. PGT fits seamlessly into this paradigm by allowing for the selection of embryos that are free from specific genetic disorders.
As the healthcare industry increasingly shifts towards personalized approaches, the demand for PGT is expected to rise. This trend is driven by advancements in genomics, which enable a deeper understanding of the genetic basis of diseases.
With personalized medicine, genetic information can be used to predict an individual's susceptibility to certain conditions, customize treatment plans, and improve health outcomes.
PGT extends these benefits to the reproductive process, providing prospective parents with the ability to select embryos that are most likely to result in healthy pregnancies and reduce the risk of inherited genetic disorders.
The growing consumer interest in personalized health solutions is fostering a market environment that supports innovation in PGT. Companies specializing in genetic testing and reproductive health are investing in research and development to create sophisticated and accessible PGT technologies.
The integration of artificial intelligence and machine learning in genetic analysis is further enhancing the accuracy and efficiency of PGT making it an attractive option for prospective parents. As healthcare providers and consumers increasingly recognize the value of personalized medicine, the PGT market stands to benefit significantly.
By offering tailored reproductive solutions that align with the principles of precision health, PGT can expand its reach and impact addressing the evolving needs and expectations of modern healthcare. This integration represents a substantial opportunity for growth and innovation in the PGT market promising better health outcomes for future generations.
The market is characterized by intense competition among key players striving to innovate and expand their market presence. Leading companies include Illumina, Thermo Fisher Scientific, Agilent Technologies, and PerkinElmer, which dominate due to their extensive portfolios in genetic testing and strong research and development capabilities.
Emerging firms like CooperSurgical and Natera have also made significant strides with novel technologies and strategic collaborations. Also, frequent mergers and acquisitions, partnerships with IVF clinics, and ongoing advancements in genetic sequencing technologies mark the competitive landscape.
Recent Developments in the Preimplantation Genetic Testing Market
|
Attributes |
Details |
|
Forecast Period |
2024 to 2031 |
|
Historical Data Available for |
2019 to 2023 |
|
Market Analysis |
US$ Billion for Value |
|
Key Regions Covered |
|
|
Key Market Segments Covered |
|
|
Key Companies Profiled in the Report |
|
|
Report Coverage |
|
|
Customization & Pricing |
Available upon request |
By Procedure
By Product
By Technology
By Region
To know more about delivery timeline for this report Contact Sales

The market is estimated to exhibit a CAGR of 9% during the forecast period.
North America is a leading regional market.
A key opportunity lies in the integration of personalized medicine and precision health.
A few of the leading companies in the market are Quest Diagnostics Incorporated, Natera, Inc., and COOPER SURGICAL.
Next Generation Sequencing is the dominant technology segment.