Pancreatic and Biliary Stents Market Segmentation by Plastic Pancreatic and Biliary Stent, Metal Pancreatic and Biliary Stent, Non-Covered Pancreatic and Biliary Stents, Metal Partially Covered Pancreatic and Biliary Stents, Metal Fully Covered Pancreatic and Biliary Stents
Industry: Healthcare
Published Date: May-2019
Format: PPT*, PDF, EXCEL
Delivery Timelines: Contact Sales
Number of Pages: 245
Report ID: PMRREP28561
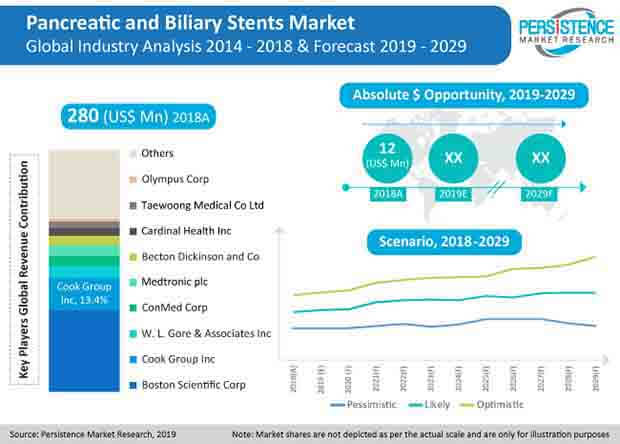
The global pancreatic and biliary stents market is fragmented in nature, and is characterized by oligopolistic competition among few renowned brands in the market. Following the patent pool in the pancreatic and biliary stents market, established manufacturers are exploring newer opportunities for revenue generation.
Considering the growth prospects in emerging markets, various companies, such as Cook Medical Inc., Boston Scientific Corporation, CONMED Corporation, and several others, are competing to capture the maximum share in emerging markets. Marketplace discussions related to pancreatic and biliary stents indicate that pancreatic cancer and benign biliary strictures applications exhibit large untapped opportunities for the manufacturers of Pancreatic and Biliary Stents, and this trend is widely observed in the U.S. pancreatic and biliary stents market in ambulatory care centres and cancer care centres.
Manufacturers in the global pancreatic and biliary stent market are making major investments and are focusing on introducing innovative products to enhance their market shares. For instance, in January 2019, AMG International GmbH received the European CE mark approval for its ARCHIMEDES biodegradable biliary and pancreatic stents. In March 2012, WallFlex Biliary Transhepatic stent system was launched by Boston Scientific Corporation in CE-marked countries for the treatment of benign biliary strictures.
Moreover, some companies, as a part of their strategy to capture a larger market share, are launching pancreatic and biliary stents with additional features. For example, W. L. Gore & Associates, Inc. launched VIABIL Short Wire Biliary Endoprosthesis with a unique antimigration technology that reduces the preoperative procedure. Rapid industrialization in emerging economies in the East African belt, Eastern Europe, and Asia Pacific regions is supporting the production and R&D of low-cost medical devices.
The availability of a well-developed manufacturing infrastructure, low-cost labour, and trained technicians in countries such as China are also among factors supporting the growth of the market. Companies that manufacture pancreatic and biliary stents, such as Taewoong Medical and Boston Scientific, have R&D centres as well as manufacturing divisions based in developing and developed economies such as Ireland, Malaysia, and China. Not only is this strategy profitable, but also results in an increase in the sales of pancreatic and biliary stents in these high-growth markets.
Advancements in gastrointestinal surgeries during the past few decades have increased the number of the options for the treatment of pancreatic and biliary diseases. The risks associated with gastrointestinal treatment are lower as compared to traditional open surgical treatments.
Surgery was the primary treatment for biliary obstruction prior to the use of stents. The use of pancreatic and biliary stents are the mainstay for pancreas divisum therapy, along with new technologies such as bioabsorbable stents, which has increased the options for the treatment of complex lesions via open surgery.
The constant development of new innovations and intervention techniques for the treatment of pancreatic diseases is estimated to increase the number of pancreatic and biliary surgeries. These factors are leading to an increase in the number of hospitals floating tenders for the procurement of better-quality pancreatic and biliary stents.
This trend is being observed in developed and developing markets in North America, Asia Pacific, and Europe. Thus, growing awareness regarding stent-based treatments is expected to fuel the growth of the pancreatic and biliary stents market during the forecast period.
In some cases, the usage of biliary or pancreatic stents in the treatment of biliary disorders and pancreatic cancer leads to various complications and can cause potential blockage. Both these types of stents can cause blockages or adverse effects such as aching muscles, high temperature, tummy pain, and other symptoms. There is also the risk of getting a hole in the duodenum during or after the procedure of stent placement in the duct, which is likely to cause bleeding and infection.
Some of the potential complications associated with the usage of a biliary metal stent placement/ERCP procedure are pain, bleeding, fever, nausea, vomiting, and acute cholangitis associated with CBD stones.
Moreover, some of the major complications associated with pancreatic and biliary stents are stent displacement or misplacement, perforation, obstruction, collapse, stent fracture, and certain infections. Such complications are likely to hamper the demand for pancreatic and biliary stents throughout the forecast period.
Pancreatic and biliary stents are used to facilitate the drainage of bile into the digestive tract, mostly in biliary obstruction, biliary fistulas, and biliary strictures. Basically, biliary stents are the currently available endoscopic solutions for stent insertion. The placement of a pancreatic and biliary stent is nearly a new and frequently adopted method to reduce the risk of post-endoscopic retrograde cholangiopancreatography.
PMR conducted a research study on the pancreatic and biliary stents market for the forecast period 2019 to 2029.
The report offers a comprehensive evaluation of the business opportunities prevailing in the pancreatic and biliary stents market, along with insights on the pancreatic and biliary stents market value analysis based on the region, global & regional market trends, pricing analysis based on product type, regulatory scenario, macro-economic factors, industry insights, pricing analysis by vendors, new disease indication forecast analysis, hazard analysis, and volume analysis by vendors.
The Pancreatic and biliary stents market report elaborates the macro-economic factors influencing the dynamics of the market and its futuristic potential.
The report on the pancreatic and biliary stents market offers a comprehensive taxonomy of the market for Pancreatic and Biliary Stents based on product type, application, end user, and region.
By product type, the pancreatic and biliary stents market is segmented into plastic pancreatic and biliary stent and metal pancreatic and biliary stent. Metal pancreatic and biliary stent is further subdivided into metal non-covered pancreatic and biliary stents, metal partially covered pancreatic and biliary stents, and metal fully covered pancreatic and biliary stents.
By application, the pancreatic and biliary stents market is segmented into benign biliary strictures, biliary leaks, malignant obstruction, biliary stones, and pancreatic cancer.
By end user, the pancreatic and biliary stents market is segmented into hospitals, ambulatory surgical centers, and specialty clinics.
By region, the Pancreatic and Biliary Stents market is segment into North America, Latin America, Europe, East Asia, South Asia, Oceania, and the Middle East & Africa.
Some of the additional questions addressed in this report on the pancreatic and biliary stents market are:
To know more about delivery timeline for this report Contact Sales
