Industry: Healthcare
Published Date: January-2025
Format: PPT*, PDF, EXCEL
Delivery Timelines: Contact Sales
Number of Pages: 183
Report ID: PMRREP16795
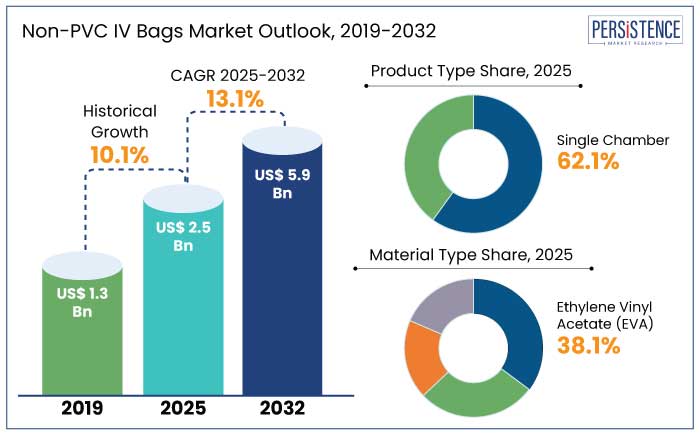
The global non-PVC IV bags market is estimated to reach a size of US$ 2.5 Bn in 2025. The industry is predicted to exhibit a CAGR of 13.1% during the forecast period to attain a value of US$ 5.9 Bn by 2032.
Concerns regarding environmental hazards associated with PVC-based medical devices have led to a shift toward non-PVC IV bags. Rising prevalence of chronic diseases like cancer, diabetes, and kidney disorders are boosting the demand for intravenous therapies.
Regulatory bodies like the FDA and EMA emphasize the need for safer drug delivery systems to minimize patient exposure to harmful substances like DEHP (di(2-ethylhexyl)phthalate), commonly found in PVC. Increasing adoption of non-PVC materials complies with these regulations, driving growth.
Key Highlights of the Market
|
Market Attributes |
Key Insights |
|
Global Non-PVC IV Bags Market Size (2025E) |
US$ 2.5 Bn |
|
Projected Market Value (2032F) |
US$ 5.9 Bn |
|
Global Market Growth Rate (CAGR 2025 to 2032) |
13.1% |
|
Historical Market Growth Rate (CAGR 2019 to 2024) |
10.1% |
Non-PVC IV Bags Market in North America is predicted to hold a share of 46.7% in 2025. Growing need for safer and sustainable medical products is driving expansion.
In the U.S., stringent regulations aimed at enhancing patient safety facilitate the adoption of these bags as they don't contain hazardous plasticizers like DEHP, decreasing the chance of chemicals leaking in IV fluids. As healthcare procedures become environmentally conscientious, non-PVC IV bags, which are lightweight and recyclable, are gaining popularity.
Technological advancements and stringent regulation promote the use of non-toxic products in the healthcare sector. Important end users like clinics, hospitals, and ambulatory surgery centers ensure steady growth.
Single chamber is predicted to hold a share of 62.1% in 2025. Its extensive use in intravenous therapy and fluid management further propel growth. These bags are widely used in hospitals, clinics, and ambulatory care facilities for delivering saline, glucose, and other vital solutions owing to their affordability and ease of use.
Simple design of single chamber bags decreases the chance of errors during preparation and administration, ensuring better patient safety. Increasing demand for easily navigable and disposable medical equipment encourages their use.
New manufacturing techniques have made single chamber bags more robust and compatible with various medical solutions. As healthcare facilities place a high priority on cost containment and efficiency, the demand for single chamber sector is likely to continue expanding in the highly competitive industry.
Ethylene vinyl acetate (EVA) is anticipated to hold a share of 38.1% in 2025. Its exceptional properties facilitate expansion. EVA is known for its robust, flexible, and chemical-resistant properties, making it a popular material for IV bags that hold a range of medical fluids. It’s biocompatible and non-toxic features are well-suited for sensitive applications, including cancer therapies and parenteral nutrition.
EVA's ability to tolerate high temperatures without losing shape or integrity is another factor bolstering demand. Rising need for eco-friendly alternatives to PVC have prompted the adoption of EVA-based solutions.
Liquid mixture is estimated to hold a share of 79% in 2025. Parental nutrition (PN) solutions are widely used in critical care and for patients that are unable to consume food orally.
Non-PVC IV bags provide a safer storage option for these sensitive mixtures, as they eliminate the risk of chemical leaching. Chronic diseases like diabetes, cancer, and kidney disorders often require intravenous therapies involving liquid mixtures.
Liquid mixtures are frequently stored in multi-chamber IV bags, which allow for the separation of components until use, ensuring stability and efficacy. Non-PVC materials are better suited for these bags owing to their flexibility and resistance to interaction with contents.
The non-PVC IV bags market is gaining traction owing to the rising demand for eco-friendly and patient-safe intravenous solutions. These bags are crafted from materials like polypropylene (PP) or polyethylene (PE), eliminating the risks associated with polyvinyl chloride (PVC) and plasticizers such as DEHP.
IV bags made from non-PVC materials offer enhanced compatibility with sensitive drugs, decrease the risk of contamination, and minimize environmental impact during disposal. Their increased use in clinics and hospitals owing to stricter regulations along with a growing desire for sustainable healthcare practices propel growth.
The non-PVC IV bags market growth was robust at a CAGR of 10.1% during the historical period. Serious health and environmental concerns regarding polyvinyl chloride (PVC) encouraged the adoption of non-PVC substitutes.
Stringent regulations along with growing patient safety consciousness is driving expansion in the industry. Enhanced compatibility of non-PVC IV bags with delicate medications, and lower risk of contamination make them a go-to choice in developed nations.
The forecast period is predicted to witness increased adoption owing to the rising incidence of chronic illnesses, improvements in drug delivery technologies, and a global movement toward sustainable medical practices. Technological advancements in material science is predicted to further improve the functionality of non-PVC IV bags.
Increasing Demand for Safe Drug Delivery
Healthcare professionals are prioritizing alternatives that guarantee patient safety owing to the increased awareness of the negative consequences of traditional polyvinyl chloride (PVC) bags. No chance of chemical leaching makes non-PVC IV bags ideal for use with delicate medications like parenteral feeding solutions, biologics, and chemotherapeutic medicines.
Increased demand for cutting-edge drug delivery systems that preserve medication efficacy while decreasing contamination hazards further propel growth. Adoption of non-PVC IV bags is further fueled by their excellent barrier qualities, which guarantee the stability of complicated drug compositions.
Rising Awareness of Patient Safety and Environmental Concerns
Increased awareness of patient safety and environmental issues is primarily driving the demand for non-PVC IV bags. As consumers and healthcare professionals learn about the possible harmful effects of PVC-based medical devices, safer alternatives are becoming gaining popularity. Polypropylene (PP) or polyethylene (PE) non-PVC IV bags provide a more flexible and safer alternative, eliminating these health risks and improving treatment outcomes.
Rising global focus on environmental sustainability has prompted healthcare systems to rethink their use of disposable products. PVC makes up a large portion of the plastic waste generated in healthcare environments. Since non-PVC alternatives have a smaller environmental impact, they align with the medical industry's increasing desire for environmentally responsible procedures.
Challenges In Maintaining Sterilization Standards For Non-PVC Materials
Adoption of non-PVC IV bags is severely hampered by the difficulty of upholding strict sterilizing regulations for non-PVC materials. In contrast to conventional PVC bags, specific sterilization processes are needed for non-PVC materials like polypropylene or polyethylene, which may be sterilized readily using tried-and-true techniques like ethylene oxide (ETO) or gamma irradiation.
These techniques can be expensive, thereby increasing production costs and restricting their widespread application in medical institutions. Concerns regarding total microbiological safety are also raised, further hampering demand. Ensuring constant sterilization while maintaining the usefulness and integrity of non-PVC IV bags is a major concern that could affect their use in high-risk clinical settings.
Demand For Multilayer Films in IV Bag Production
Growing need for multilayer films offers a chance to develop new medical packaging options. These films are prized for their exceptional barrier qualities, which shield intravenous fluids from moisture, light, and oxygen while maintaining their integrity. Stability and safety of medications are guaranteed by this improved protection.
As healthcare infrastructure expands globally, there is an increasing demand for clean and dependable packaging solutions, driving the use of multilayer films in IV bag manufacturing. Manufacturers are progressively adopting these technologies to satisfy the changing needs of contemporary drug delivery and healthcare systems.
Integration with Advanced Infusion Systems
Combination of non-PVC IV bags with advanced infusion systems presents a significant opportunity for growth. This expansion is likely to be driven by the rising need for precision and safety in drug administration.
Innovative infusion systems with sensors, automatic dosage, and real-time monitoring are gaining traction as medical professionals work on decreasing pharmaceutical errors while enhancing treatment efficacy. Non-PVC materials, valued for their excellent compatibility with a wide range of medications, are a reliable choice when integrated with these sophisticated infusion technologies.
The competitive landscape of the global non-PVC IV bags market is dominated by prominent players who prioritize sustainability, innovation, and regulatory compliance. To provide safer and more dependable IV bags, top manufacturers are utilizing cutting-edge materials including polypropylene and polyethylene.
Companies like Baxter International and Renolit are known for their high-quality and eco-friendly solutions. Businesses are collaborating with healthcare providers to integrate non-PVC IV bags with smart infusion systems. As the need for safer drug delivery methods grows, businesses are competing fiercely to provide unique and patient-focused products that adhere to changing healthcare laws.
Recent Developments in the Global Non-PVC IV Bags Market
|
Attributes |
Details |
|
Forecast Period |
2025 to 2032 |
|
Historical Data Available for |
2019 to 2024 |
|
Market Analysis |
US$ Billion for Value |
|
Key Regions Covered |
|
|
Key Market Segments Covered |
|
|
Key Companies Profiled |
|
|
Report Coverage |
|
|
Customization & Pricing |
Available upon request |
By Product Type
By Material Type
By Content
By Region
To know more about delivery timeline for this report Contact Sales

The global non-PVC IV bags are estimated to attain a value of US$ 5.9 Bn by 2032.
Growing demand for safer and sustainable medical packaging along with regulatory shifts and healthcare advancements drive growth.
Leading players include Baxter, Renolit, and B. Braun Melsungen AG.
The market is projected to record a CAGR of 13.1% through 2032.
The shift towards eco-friendly materials and innovations in sustainable and cost-effective IV bag alternatives presents numerous opportunities.