Comprehensive Snapshot of Neurological Biomarkers Market Report Including Regional and Country Analysis in Brief.
Industry: Healthcare
Published Date: April-2025
Format: PPT*, PDF, EXCEL
Delivery Timelines: Contact Sales
Number of Pages: 170
Report ID: PMRREP16303
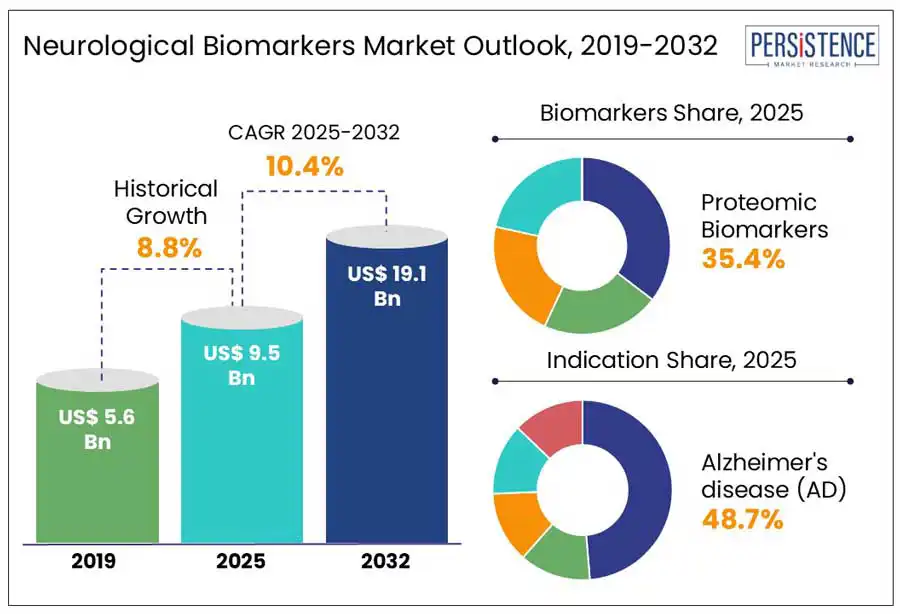
According to the Persistence Market Research, the global neurological biomarkers market value is estimated to grow from US$ 9.5 Billion in 2025 to US$ 19.1 Billion by 2032. The market is projected to record a CAGR of 10.4% by 2032. The rising prevalence of neurological disorders and advances in molecular biology, such as gene sequencing and proteomics, alongside neuroimaging techniques are expanding the application of biomarkers in diagnostics and treatment. According to the World Health Organization, the global burden of neurological diseases is expected to increase substantially, further driving demand for early diagnostic tools.
Key Industry Highlights:
|
Global Market Attribute |
Key Insights |
|
Neurological Biomarkers Market Size (2025E) |
US$ 9.5 Bn |
|
Market Value Forecast (2032F) |
US$ 19.1 Bn |
|
Projected Growth (CAGR 2025 to 2032) |
10.4% |
|
Historical Market Growth (CAGR 2019 to 2024) |
8.8% |
Advancements in genomics and proteomics have notably accelerated the discovery of neurological biomarkers, improving the diagnosis and treatment of neurological disorders. Genomics and proteomics identify gene mutations and protein biomarkers aiding early diagnosis and personalized treatments, improving outcomes in neurodegenerative diseases. Advancements in next-generation sequencing (NGS) and mass spectrometry are further accelerating genomics and proteomics discoveries by identifying genetic variants such as APOE, APP, PSEN1, and PSEN2 linked to Alzheimer’s risk. Growing usage in neurological disease research is driving the global market.
In January 2025, the UK Biobank in collaboration with 14 pharmaceutical companies launched a 2025 proteomics initiative that analyzes blood samples from 500,000 participants to identify up to 5,400 proteins. This project uses AI to enhance disease prediction, treatment targeting, and overall understanding of neurological conditions.
Clinical trials for validating biomarkers are time-consuming and costly, often needing large patient cohorts to ensure statistical significance. This is particularly challenging in neurological diseases, where patient recruitment can be difficult, and disease progression may vary widely. As a result, many biomarkers fail to meet the rigorous standards by regulatory bodies, such as the FDA or EMA. Additionally, there is a lack of standardized protocols for biomarker testing in clinical settings, which leads to inconsistencies in results. The variability of biomarkers in the population further complicates the establishment of universally accepted standards. Despite significant progress in the discovery of neurological biomarkers challenges clinical validation, thus slowing their widespread adoption.
AI and ML presents a major opportunity for researchers and biotech firms by enabling rapid analysis of complex genomic, proteomic, and imaging data. These technologies help identify novel neurological biomarkers, predict disease progression, and tailor treatments. Their ability to process large datasets efficiently accelerates discovery, supports personalized medicine, and shortens time-to-market for diagnostics and therapies. In December 2023, Quibim, a pioneer in imaging biomarkers for Precision Medicine, launched QP-Brain® after receiving FDA 510(k) clearance, a CE mark for the EU, and a UKCA mark for the UK, enabling its clinical use.
Proteomics biomarkers accounted for 35.4% share in 2024, Proteomic biomarkers are preferred over genomic or metabolomics counterparts due to their higher clinical relevance in reflecting disease phenotype. Unlike genomic biomarkers, which indicate static risk factors, proteomic markers capture dynamic biological responses such as protein misfolding or aggregation central to neurological disorders. Their closer correlation with pathophysiological changes enables more accurate disease staging, early intervention, and real-time treatment monitoring. This translational advantage makes proteomics a more effective tool for guiding therapeutic decisions and developing targeted neurology drugs, driving their prioritization in biomarker research and clinical pipelines.
Alzheimer's disease (AD) is projected to account for over 45% value share within the global market in 2025. High prevalence of Alzheimer is creating significant demand for diagnostic tools for early diagnosis and effective treatment. According to Alzheimer's Association 2025, one in nine people aged 65 and above suffer from Alzheimer's.
Introduction of blood-based biomarker testing and imaging techniques has refined the diagnosis of AD and guided treatment decisions, especially as disease-modifying therapeutics become more prevalent. Biomarkers such as tau proteins and amyloid-beta are crucial for identifying AD in its early stages, enabling the initiation of AD-targeted therapies.
In contrast, other neurological conditions like multiple sclerosis (MS), Parkinson's disease (PD), and Autism spectrum disorder (ASD) still have fewer well-established biomarkers, limiting their market growth relative to Alzheimer's. This disparity in biomarker development further solidifies AD’s dominant position within the global market.
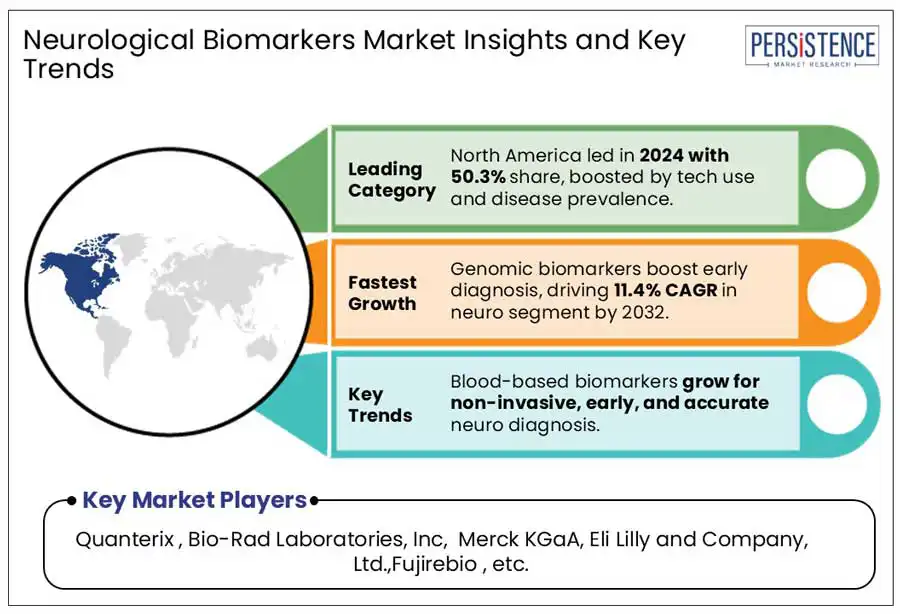
North America accounted for 50.3% of the global market in 2024, with the U.S. emerging as a key player due to the rising prevalence of neurodegenerative diseases such as Alzheimer’s and Parkinson. According to Alzheimer Association, around 6.9 million American populations were affected by Alzheimer’s in 2024, a number projected to rise to 13.8 million by 2060, significantly increasing demand for early diagnostic tools.
Strong emphasis on advancing biomarker research, active research programs and substantial R&D funding are significantly propelling the North American market. The region’s commitment to developing novel biomarkers drive advancements in the early diagnosis and personalized treatment approaches for neurological disorders.
In January 2024, the U.S. National Institutes of Health (NIH) launched a major initiative to refine NFL assay performance, aimed at advancing early Alzheimer’s detection. This reflects a growing investment in biomarker innovation to support timely intervention and disease management.
The growing use of biomarkers in clinical trials and FDA’s Biomarker Qualification Program is accelerating drug development by accepting biomarkers as surrogate endpoints accelerating the approval process for new neurological treatments. The incorporation of artificial intelligence (AI) into biomarker analysis is enhancing diagnostic accuracy, further driving the U.S. neurological biomarker market. Companies such as C2N Diagnostics have pioneered AI-driven blood tests, such as the PrecivityAD2 test (UK MHRA medical device certification in February 2025), which measures amyloid-beta levels to aid in Alzheimer’s disease diagnosis.
In 2024, Europe held a 24.6% share. Rise in research funding and demand for early diagnosis of conditions such as Alzheimer’s, Parkinson’s, and multiple sclerosis. Advanced blood biomarkers such as p-Tau217 and p-Tau181 is developed to detect these diseases at earlier stages. European governments and private institutions are heavily investing in neuroscience, resulting in specialized research centers and cross-border collaborations.
The UK government allocated an additional US$ 2.5 million in March 2025 for the READ-OUT trial, that focuses on blood biomarkers for early dementia detection. This reflects a wider regional push to enhance diagnostic accuracy and improve patient outcomes through early intervention.
Asia Pacific accounted for 13.2% of the global market in 2024. Asia Pacific's growing disease burden is boosting biomarker innovation. The region faces increasing cases of stroke, dementia, and Parkinson’s disease, further fueling demand for early diagnostics.
India neurology biomarker market is witnessing rapid growth, owed to increasing incidence of neurological conditions alongside government programs such as NPCDCS (National Programme for Prevention and Control of Cancer, Diabetes, Cardiovascular Diseases and Stroke) that promote advanced diagnostic technologies.
China’s “Healthy China 2030” initiative focuses on early detection and healthcare strengthening. Alzheimer’s Disease International projects China to have over 20 million dementia cases by 2050. These trends highlight the need for scalable diagnostic infrastructure. With advancing technology and a supportive research environment, Asia Pacific is becoming a key player in neurological biomarker innovation.
The global neurological biomarkers market is highly fragmented, with key players focusing on innovation and product development through strategic partnerships. Advancements in biomarker technologies and the growing demand for early disease detection is fueling market expansion and enhancing competitive positioning.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis |
Value: US$ Mn/Bn Volume: As applicable |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Biomarkers Type
By Sample
By Indication
By End-user
By Region
To know more about delivery timeline for this report Contact Sales

The global market is projected to value at US$ 9.5 Bn in 2025.
The global market is driven by rising neurodegenerative disease prevalence, and rapid adoption of advanced diagnostic and biomarker technologies.
The global market is poised to witness a CAGR of 10.4% from 2025 to 2032.
Emerging non-invasive biomarkers and increasing applications in personalized medicine and early neurological disease detection.
The key players in the neurological biomarkers market include Quanterix, Amoneta Diagnostics, Bio-Rad Laboratories Inc. Fujirebio, Merck KGaA, and others.