Industry: Healthcare
Published Date: July-2024
Format: PPT*, PDF, EXCEL
Delivery Timelines: Contact Sales
Number of Pages: 195
Report ID: PMRREP34631
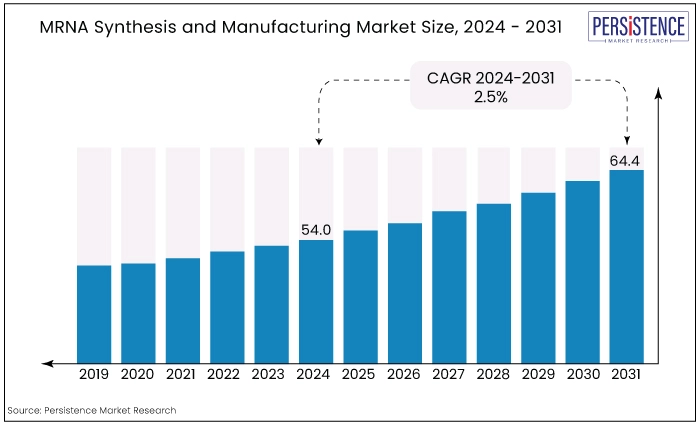
The global mRNA synthesis and manufacturing market size is estimated to value at US$64.4 Bn by the end of 2031, up from US$52.7 Bn recorded in 2023. The market is expected to secure a CAGR of 2.5% in the forthcoming years from 2024 to 2031.
Key Highlights of the Market
|
Market Attributes |
Key Insights |
|
MRNA Synthesis and Manufacturing Market Size (2024E) |
US$54.0 Bn |
|
Projected Market Value (2031F) |
US$64.4 Bn |
|
Forecast Growth Rate (CAGR 2024 to 2031) |
2.5% |
|
Historical Growth Rate (CAGR 2018 to 2023) |
2.2% |
The market for mRNA synthesis and manufacturing encompasses the production and development of messenger RNA molecules used in vaccines and therapeutic applications.
mRNA is a molecule that plays a crucial role in the body's cellular processes by carrying genetic information from DNA to the ribosomes, where proteins are synthesized.
In recent biomedical applications, mRNA has gained attention as a promising tool for therapeutic interventions and vaccine development due to its ability to instruct cells to produce proteins that can trigger immune responses or correct genetic defects.
The global market has seen significant growth due to breakthroughs in COVID-19 vaccines, highlighting mRNA's potential for rapid response to infectious diseases.
Historically, the mRNA synthesis and manufacturing industry has experienced exponential growth, propelled by breakthroughs in vaccine development.
The success of COVID-19 vaccines underscored mRNA's potential in rapidly addressing global health challenges. This has spurred increased investment in research, technology, and infrastructure to enhance production capabilities and expand therapeutic applications beyond infectious diseases.
Looking ahead, the market is poised for continued expansion as companies focus on refining manufacturing processes, optimizing delivery systems, and broadening the scope of mRNA therapies.
Key areas of development include cancer vaccines, personalized medicine, and treatments for rare genetic disorders. Regulatory agencies' support and streamlined approval processes are expected to facilitate market growth further.
Innovations in mRNA synthesis, such as improved stability and scalability, will be critical in overcoming current challenges related to manufacturing complexity and cost.
Growing strategic collaborations between biopharmaceutical companies, academic institutions, and government bodies is expected play a crucial role in driving innovation and shaping the future landscape of mRNA-based therapies and vaccines.
Continuous Innovation in mRNA Synthesis and Manufacturing Techniques
The rapid advancements in mRNA technology are revolutionizing the biopharmaceutical industry. Innovations in mRNA synthesis and manufacturing, particularly the development of sophisticated lipid nanoparticle delivery systems, are pivotal in driving market growth.
Such advancements enhance the stability, efficacy, and delivery efficiency of mRNA-based therapeutics and vaccines.
The ability to precisely engineer mRNA sequences and optimize delivery mechanisms has significantly expanded the therapeutic potential of mRNA technology beyond vaccines, into treatments for various diseases like cancer, genetic disorders, and autoimmune conditions.
Continuous innovation not only improves patient outcomes but also attracts substantial investment and collaboration across industry sectors, fostering a robust ecosystem for further development and commercialization.
Growing Collaborations Among Industry Players
The market for mRNA synthesis and manufacturing is witnessing a surge in collaborations among industry players, driven by the rapid advancements and promising applications in healthcare.
Companies are pooling resources to enhance production capabilities, streamline processes, and accelerate innovation.
Collaborations span across research and development partnerships, joint ventures for manufacturing facilities, and technology sharing agreements. These efforts aim to meet the increasing demand for mRNA-based therapies and vaccines, ensuring scalability and reliability in production.
By leveraging collective expertise and infrastructure, industry players are poised to make significant strides in addressing global health challenges and expanding the therapeutic potential of mRNA technology.
Complexity of Manufacturing Steps
The complexity of manufacturing steps poses a significant restraint for mRNA synthesis and manufacturing. The process involves intricate procedures such as mRNA synthesis, purification, and formulation with lipid nanoparticles, each requiring precise control and specialized equipment.
Ensuring consistency and scalability across production batches adds further challenges. Moreover, stringent regulatory requirements for quality control and validation increase operational complexities and costs. These factors collectively limit the speed and scale of production, potentially delaying time-to-market for new therapies.
Addressing these challenges necessitates ongoing investment in automation, process optimization, and regulatory compliance strategies to streamline manufacturing processes and meet growing global demand effectively.
Limited Accessibility of GMP-Grade Raw Materials for mRNA Manufacturing
GMP-grade materials are essential to ensure the safety, efficacy, and quality of mRNA therapeutics and vaccines.
However, the availability of these materials can be constrained due to stringent regulatory requirements, limited suppliers capable of meeting GMP standards, and the specialized nature of raw materials required for mRNA synthesis and formulation.
This scarcity can lead to supply chain disruptions, increased costs, and delays in production timelines. Addressing this challenge involves expanding the supplier base, improving logistics, and fostering partnerships to ensure reliable access to high-quality GMP-grade raw materials necessary for manufacturing mRNA-based products.
The limited accessibility of Good Manufacturing Practice (GMP)-grade raw materials, thus, presents a significant restraint in the mRNA manufacturing sector.
Regulatory Support, Accelerated Approvals, and Multiple Funding Initiatives
Regulatory support and accelerated approvals are crucial for advancing the market for mRNA synthesis and manufacturing. Close collaboration between industry pioneers and regulatory bodies is essential to establish robust guidelines and expedite approval processes for mRNA-based therapies.
The proactive engagement not only accelerates the time to market for new treatments but also ensures stringent safety and efficacy standards are met.
Furthermore, funding initiatives undertaken by regulatory bodies plays a pivotal role in accelerating the development and production of mRNA technologies.
Such initiative not only supports innovative advancements in vaccine and therapeutic development but also reinforces the FDA's commitment to fostering a robust ecosystem for mRNA technologies.
Growing Opportunities in mRNA Vaccines and Therapeutics
The rising expectations surrounding mRNA vaccines and therapeutics present a significant opportunity for the biopharmaceutical industry. These expectations are driven by mRNA's potential to revolutionize vaccine development timelines, offering rapid response capabilities to emerging infectious diseases and variants.
Moreover, there is growing interest in expanding mRNA applications beyond vaccines into areas such as cancer immunotherapy, infectious diseases, and personalized medicine.
The shift is fuelled by promising clinical trials and early successes in targeting specific diseases with mRNA technology. As a result, pharmaceutical companies are increasingly investing in research and development, alongside scaling up production capacities to meet anticipated global demand.
The convergence of technological advancements, regulatory support, and public acceptance creates a fertile ground for innovation in mRNA therapies. This presents an opportune moment for stakeholders to capitalize on the momentum, driving forward the next generation of medical treatments that harness mRNA's transformative potential.
|
Category |
Projected CAGR through 2031 |
|
Scale of Operation – Preclinical |
2.10% |
|
Application- Vaccine Production |
2.40% |
Preclinical Segment to Account for the Significant Share
The preclinical operation segment dominated the global market in 2023 and is likely to maintain its dominance during in the forthcoming years recording a CAGR of 2.10% due several factors.
Preclinical studies are essential for assessing the safety, efficacy, and potential toxicity of mRNA-based therapies and vaccines before progressing to clinical trials. Robust preclinical data are necessary to secure regulatory approvals and attract investment for further development.
Moreover, early-stage research allows for optimization of mRNA constructs, delivery systems, and manufacturing processes, laying a solid foundation for successful clinical translation.
The preclinical segment therefore not only influences strategic decision-making but also mitigates risks associated with later-stage development, ensuring that mRNA products meet rigorous standards before advancing to human trials and commercialization efforts.
Vaccine production account for a significant share in the global industry. The success of mRNA vaccines like those for COVID-19 has demonstrated the rapid development and scalability advantages of this technology in responding to global health crises.
The scenario has increased confidence and investment in mRNA for vaccine applications, driving significant market demand. Additionally, ongoing research aims to expand mRNA vaccines to target a broader range of infectious diseases, further bolstering their market relevance.
The established infrastructure and regulatory pathways from vaccine production also facilitate easier entry into other therapeutic areas, such as cancer immunotherapy and genetic disorders, enhancing the overall versatility and attractiveness of mRNA technology in the biopharmaceutical industry.
|
Region |
CAGR through 2034 |
|
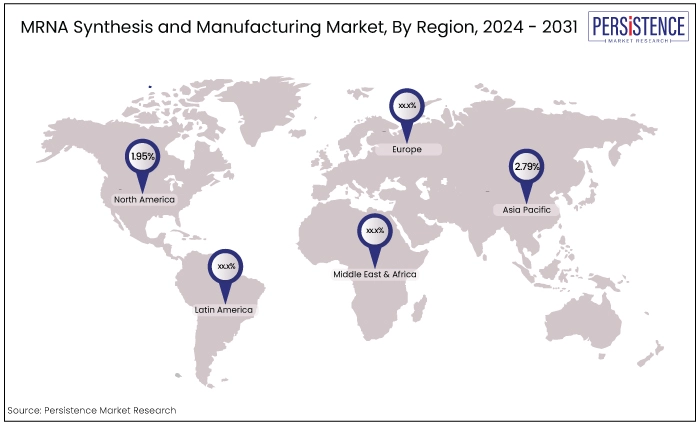
North America |
1.95% |
|
East Asia |
2.79% |
North America Secures the Leading Position
North America is a significant shareholder in the global market and is expected to grow at a CAGR of 1.95% during the forecast period. The region boasts a robust biopharmaceutical industry with a strong emphasis on innovation and research, particularly in mRNA technology.
Major companies pioneering in mRNA vaccines, are based in North America, driving technological advancements and setting industry standards.
Moreover, North America benefits from supportive regulatory frameworks that facilitate faster approvals for mRNA-based therapies and vaccines. The FDA's proactive approach in approving COVID-19 mRNA vaccines highlighted its commitment to advancing cutting-edge biotechnologies.
Additionally, the region attracts substantial investments in healthcare infrastructure and R&D, fostering a conducive environment for biotech startups and partnerships. These factors collectively position North America as the leader in mRNA synthesis and manufacturing, contributing to its significant global market share.
East Asia All Set to Exhibit a Notable CAGR
The East Asia market is projected to secure a CAGR of 2.79% during the forecast period from 2024 to 2031.
The region has been at the forefront of biotechnological innovation, with countries like China, South Korea, and Japan investing heavily in advanced healthcare technologies. Increasing focus on mRNA research and development will bring in profits to the market of East Asia.
Moreover, strategic partnerships and collaborations between East Asian biotech firms, and globally leading pharmaceutical companies are accelerating technology transfer and market penetration. This collaborative ecosystem fosters innovation and enhances competitiveness in the global biopharmaceutical market.
Expansion of manufacturing capabilities and strategic collaborations among the leading industry players are some of key strategies being undertaken to bring innovative and effective mRNA products into the market, thereby, boosting the global market for mRNA synthesis and manufacturing.
Recent Industry Developments
April 2024
TriLink BioTechnologies opened its new cGMP mRNA manufacturing facility in San Diego to support late-phase drug developers from Phase 2 to commercialization via TriLink’s robust mRNA manufacturing capabilities,
April 2024
FUJIFILM Toyama Chemical Co., Ltd entered a strategic partnership with Synplogen Co., Ltd. on CDMO services for mRNA therapeutics. The alliance is aimed to provide end-to-end services from mRNA sequence design to LNP formulation and fill/finish, ensuring consistent support throughout the drug discovery and development process.
January 2024
Lonza collaborated with Oxford Nanopore Technologies plc to cGMP validate and commercialize a first-of-its-kind novel test. The analytical test will accurately determine multiple critical quality attributes of mRNA products by directly sequencing both the DNA template and the messenger RNA (mRNA) and is expected to accelerate the path to market for mRNA products such as vaccines for infectious diseases.
September 2023
PackGene Biotech partnered with Kudo Biotechnology to provide high-quality, customized messenger RNA (mRNA) manufacturing services for drug and vaccine development.
July 2023
Ardena entered a strategic alliance with RiboPro to provide end-to-end solution for advanced mRNA and lipid nanoparticles (LNP) production.
July 2023
Vernal Biosciences partnered with Japan’s first induced pluripotent stem cell (iPSC) company, REPROCELL Inc., to provide mRNA services in Japan at scale for research and clinical applications.
July 2023
The Centre for Biologics Evaluation and Research (CBER) within the FDA provided $82 million to fund a three-year research program. The program was aimed at designing the world’s first fully integrated, continuous mRNA manufacturing platform. The program is a collaboration between ReciBioPham, to implement a pilot manufacturing site for the project, and MIT researchers and collaborators from Penn State University to tackle project engineering complexities.
|
Attributes |
Details |
|
Forecast Period |
2024 to 2031 |
|
Historical Data Available for |
2018 to 2023 |
|
Market Analysis |
US$ Million for Value |
|
Key Regions Covered |
|
|
Key Market Segments Covered |
|
|
Key Companies Profiled in the Report |
|
|
Report Coverage |
|
|
Customization & Pricing |
Available upon request |
By Service Type
By Scale of Operation
By Region
To know more about delivery timeline for this report Contact Sales

The convergence of scientific advancements, successful COVID-19 vaccines, investment inflows, and the broad therapeutic potential of mRNA technology is surging the demand for digital twin in healthcare.
Some of the key players operating in the market are DH Life Sciences, LLC (Danaher),Azenta Life Sciences (Genewiz), Merck KGaA, TriLink BioTechnologies, GenScript, Creative Biolabs, and Thermo Fisher Scientific Inc.
The vaccine production segment recorded the significant market share in 2023.
Developing mRNA-based cancer vaccines and immunotherapies using mRNA technology based on individual genetic profiles and disease characteristics is estimated to present opportunities for the market players.
North America is set to account for the significant share in the market.