Industry: Healthcare
Published Date: April-2025
Format: PPT*, PDF, EXCEL
Delivery Timelines: Contact Sales
Number of Pages: 180
Report ID: PMRREP35195
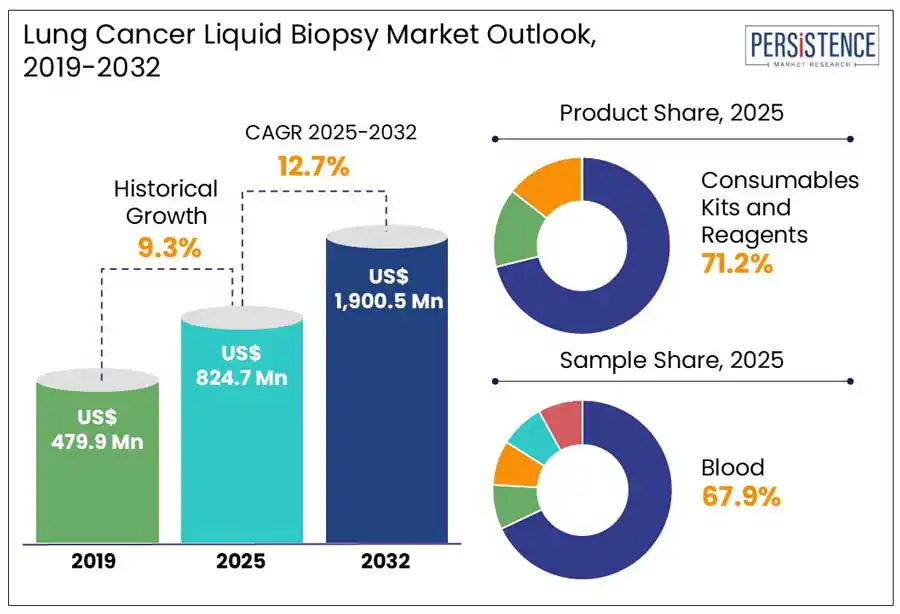
The global lung cancer liquid biopsy market size is to reach US$ 1,900.5 million from US$ 824.7 million in 2025 to grow at a CAGR of 12.7% by 2032, according to the recent report published by Persistence Market Research.
The growing prevalence of lung cancer in the population and rising demand for non-invasive diagnostic methods drives global market expansion for lung cancer liquid biopsy. Compared to the traditional tissue biopsies, liquid biopsies analyze blood-based biomarkers such as cell-free DNA (cfDNA), circulating tumor cells (CTCs) and exosomes among others. This enables the early detection of lung cancer and more personalized less invasive treatment options.
Key Industry Highlights:
|
Global Market Attribute |
Key Insights |
|
Lung Cancer Liquid Biopsy Market Size (2025E) |
US$ 824.7 Mn |
|
Market Value Forecast (2032F) |
US$ 1,900.5 Mn |
|
Projected Growth (CAGR 2025 to 2032) |
12.7% |
|
Historical Market Growth (CAGR 2019 to 2024) |
9.3% |
Biomarkers such as microRNAs, mutated genes and proteins have been used in liquid biopsy to identify early cancer presence, progression, and response to therapy. Discovery of new biomarkers plays a pivotal role in improving the specificity and accuracy of liquid biopsy tests.
In line with these advancements, BioMark Diagnostics initiated enrollment for its pivotal lung cancer screening study in January 2023, combining BioMark’s metabolomics liquid biopsy test with radiomics and genetic biomarkers to enhance lung cancer screening accessibility and accuracy in Quebec.
Similarly, Freenome launched the PROACT LUNG clinical study in December 2023 to validate its blood test for early lung cancer detection. The test integrates lung cancer-specific biomarkers with cancer-common markers from Freenome’s multiomics platform. further contributing to the evolution of more precise and personalized approaches to lung cancer diagnosis.
Technologies such as NGS and PCR microarrays has further enabled deeper genetic profiling and comprehensive molecular information, allowing early detection of lung cancer and personalized treatment strategies. Funding and collaboration initiatives are undertaken by key players to develop new technologies, expanding the range of detectable genetic alterations.
While liquid biopsy is highly demanding in cancer screening, detection, and treatment response, there are certain challenges associated with their usage such as limited sensitivity of biomarkers in detecting early-stage cancers. During early stages, the concentration of tumor-derived biomarkers, such as ctDNA or CTCs, are often very low and may produce insufficient amounts of genetic material into the blood, to be used for detection through liquid biopsy.
Complexity and diversity of tumor biology further adds to variability in biomarker expression, complicating the identification of early-stage cancers. Advancements in biomarker discovery and technology improve sensitivity, however, reaching required detection thresholds for early-stage cancers continues to be a significant hurdle, limiting the current potential of liquid biopsy as an early diagnostic tool.
Companion diagnostics (CDx) is a crucial tool gaining popularity in personalized medicine to identify lung cancer patients likely to benefit from specific targeted therapies such as EGFR inhibitors and ALK inhibitors. The capability of multigene panel assays of liquid biopsy has been approved as companion diagnostics and have been used in routine clinical settings.
Minimally invasive liquid-based CDx by liquid biopsy offers shorter turn-around time (TAT) enabling quicker, more efficient diagnostics, particularly beneficial in urgent cases. Moreover, reduction in NGS costs further supports liquid biopsy integration into routine clinical practice, making liquid biopsy an ideal CDx tool for lung cancer mutation screening.
Foundation Medicine, Inc. in November 2024, received U.S. FDA approval for FoundationOne®Liquid CDx as a companion diagnostic for TEPMETKO® (tepotinib) developed by EMD Serono, to identify patients with MET Exon 14 skipping alterations in non-small cell lung cancer (NSCLC).
This shift toward precision medicine presents a valuable opportunity for the lung cancer liquid biopsy market, fostering growth and innovation in cancer diagnostics.
Consumables kits and reagents accounted for a revenue share of 71.2% in 2024 due to their recurrent use in the diagnostic procedures. Kits and reagents necessarily collect, process, and analyse samples, making them integral to the accuracy and efficiency of liquid biopsy tests. Growing adoption of liquid biopsy technologies for lung cancer detection is projected to augment demand for consumables such as test kits, reagents, and assay components.
Globally, multi-gene-parallel analysis (NGS) dominated the technology category in 2024 with 75.2% market share due to their superior ability to analyze multiple genetic mutations simultaneously. NGS allows identification of wide genetic alterations such as point mutations, insertions, deletions, and gene amplifications, by providing comprehensive and high-throughput sequencing in lung cancer patients. NGS further enables precise targeting of therapies based on genetic profiles making them the preferred choice for both diagnostic and research applications.
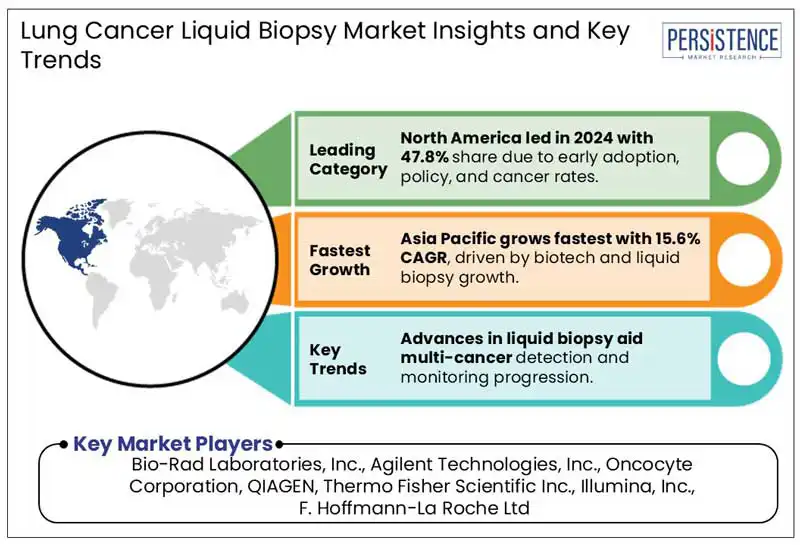
North America’s lung cancer liquid biopsy market accounted for 47.8% share in 2024. The region’s focus on widespread adoption of cutting-edge diagnostic technologies such as NGS and increased research investments fuels demand for liquid biopsy solutions. In February 2024, Roche invested USD 254 million in Freenome to advance its multiomics platform and develop blood tests for early cancer detection.
Growing prevalence of lung cancer in the U.S. significantly drive the U.S. lung cancer liquid biopsy market. As of 2025, nearly 226,650 new cases of lung cancer are estimated to be identified in the U.S. with 124,730 fatalities. 87% of these are categorized as non-small cell lung cancer (NSCLC) while small cell lung cancer (SCLC) represents 13%. Rising incidence coupled with increasing demand for less invasive diagnostic methods, positions the U.S. market for significant growth in lung cancer diagnosis and treatment.
Europe is globally the second-leading generating a significant share of 28.6% in 2024. Significant investments are made in European region to accelerate developments in liquid biopsy technologies. Rising preference for non-invasive diagnostic methods and collaborative efforts between healthcare providers, academic institutions, and biotechnology companies further fuels demand for liquid biopsy solutions for lung cancer.
In March 2024, Guardant Health partnered with The Royal Marsden to expand the use of liquid biopsy technology, providing faster, more targeted treatments for lung cancer patients through the NHS England study.
Germany’s support for cutting-edge diagnostic methods and strong regulatory framework allow for faster integration of new technologies into clinical practice. Additionally, Germany’s reimbursement policies facilitate broader access to liquid biopsy diagnostics, thereby expanding the German lung cancer liquid biopsy market.
Likewise, in the U.K. the medical professional strongly emphasize early detection and personalized treatment. Increasing integration of National Health Service (NHS) into clinical pathways and U.K. government’s investment in precision medicine and its commitment to improving cancer care, including liquid biopsy supports market expansion. The combination of research breakthroughs, regulatory support, and patient demand positions Europe as a strong contender in the global liquid biopsy market for lung cancer.
Asia Pacific is estimated to attain by 15.6% during the forecast period, driven by increasing incidence of lung cancer in the region, particularly in countries such as India, and China.
India's lung cancer liquid biopsy market is largely supported by the local biotech startups focusing on affordable, region-specific diagnostic solutions. Government initiatives aimed at enhancing early cancer detection and emphasizing cost-effective solutions are crucial factors accelerating liquid biopsy adoption in India. Johnson & Johnson launched a nationwide campaign, India Detects to Defeat, in November 2024, in collaboration with Amar Chitra Katha to assist in better understanding the risks, symptoms, and importance of early diagnosis and testing for lung cancer.
The global lung cancer liquid biopsy market is highly competitive with key players focusing on innovation and product launches through strategic research collaborations to offer advanced liquid biopsy solutions. Technological advancements, rising demand for non-invasive diagnostics, and personalized treatments drive the global market growth.
|
Attributes |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis Units |
Value: US$ Mn/Bn Volume: Units |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Product:
By Technology:
By Biomarker:
By Indication:
By Sample:
By End-user:
By Region:
To know more about delivery timeline for this report Contact Sales

The global market is set to reach US$ 824.7 Mn in 2025.
By product, consumables kits and reagents is projected to dominate the global market in 2025.
The industry is estimated to rise at a CAGR of 12.7% through 2032.
North America is projected to hold the largest share of the industry in 2025.
Bio-Rad Laboratories, Inc., Agilent Technologies, Inc., QIAGEN, Thermo Fisher Scientific Inc., Illumina, Inc., F. Hoffmann-La Roche Ltd are a few leading players.