Industry: Healthcare
Published Date: January-2025
Format: PPT*, PDF, EXCEL
Delivery Timelines: Contact Sales
Number of Pages: 184
Report ID: PMRREP17314
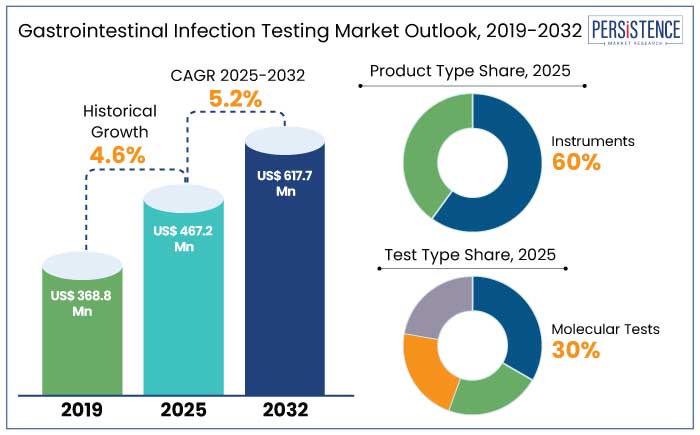
The global gastrointestinal infection testing market is predicted to reach a size of US$ 467.2 Mn by 2025. It is anticipated to witness a CAGR of 5.2% during the forecast period to attain a value of US$ 617.7 Mn by 2032.
Foodborne pathogens like Salmonella, Escherichia coli (E. coli), and Campylobacter are prominent contributors to gastrointestinal infections (GI).
Rising demand for rapid and accurate diagnostic tests is fostering the development of molecular diagnostic techniques like PCR-based and next-generation sequencing (NGS) tests. Point-of-care diagnostics that enable faster results while decreasing waiting times are gaining traction.
Key Highlights of the Industry
|
Market Attributes |
Key Insights |
|
Gastrointestinal Infection Testing Market Size (2025E) |
US$ 467.2 Mn |
|
Projected Market Value (2032F) |
US$ 617.7 Mn |
|
Global Market Growth Rate (CAGR 2025 to 2032) |
5.2% |
|
Historical Market Growth Rate (CAGR 2019 to 2023) |
4.6% |
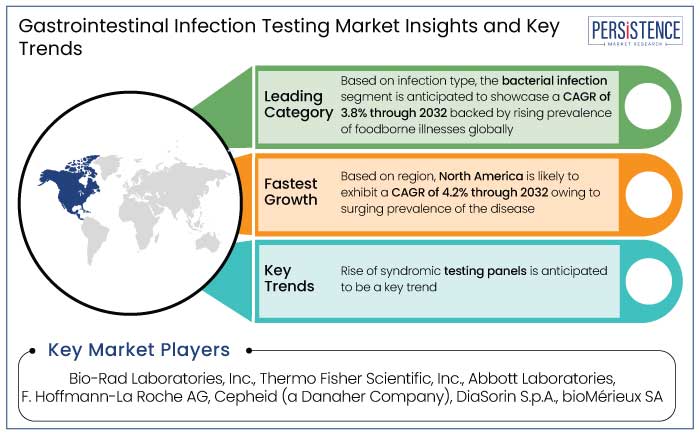
North America gastrointestinal infection testing market is estimated to hold a share of 35% in 2025. The region boasts the most unique healthcare system in the world with widespread access to healthcare services and modern diagnostic technologies. This infrastructure supports the rapid adoption of the latest gastrointestinal infection testing technologies.
Prevalence of gastrointestinal infections in North America remains high due to a combination of factors like foodborne illnesses, contamination, and outbreaks. Pathogens like Salmonella, E. coli, and Norovirus are common causes, resulting in an increasing demand for diagnostic testing to identify the causative organisms.
Instrument is anticipated to hold a share of 60% in 2025. Need for accurate and rapid diagnosis of gastrointestinal infections is one of the primary reasons behind the increasing demand for instrument-based testing. Instruments like PCR machines, immunoassay analyzers, and culture-based diagnostic tools enable high precision and speed in identifying pathogens.
Development of next-generation diagnostic instruments, like automated PCR machines and multiplex assays, has significantly improved the efficiency and accuracy of gastrointestinal infection testing. High demand for point-of-care (POC) testing is pushing the adoption of instruments that can provide quick results outside traditional laboratory settings. For example,
Molecular tests are projected to account for a share of 30% in 2025. Molecular tests, like PCR-based tests, offer high sensitivity and specificity in detecting gastrointestinal pathogens, even at very low concentrations.
Demand for rapid testing has grown owing to the need for quick identification of gastrointestinal infections, particularly in emergency settings or foodborne illness outbreaks. For instance,
The American Society for Microbiology states that rapid diagnostic tests, especially molecular tests, are vital in quickly identifying infectious agents. This assists in decreasing the length of hospital stay and preventing the overuse of antibiotics.
A BioTechniques article mentioned that multiplex PCR testing is becoming increasingly popular in clinical and commercial laboratories. Its ability to provide comprehensive pathogen identification in gastrointestinal cases spurs growth.
Potential growth in the global gastrointestinal infection testing industry is predicted to be driven by the rise in availability of over-the-counter (OTC) test kits that can be used by consumers at home to identify pathogens. This improves convenience while decreasing healthcare facility burdens. Regulatory bodies like FDA and European Medicines Agency (EMA) are streamlining approval processes for gastrointestinal infection testing devices.
The gastrointestinal infection testing market growth was steady at a CAGR of 4.6% during the historical period from 2019 to 2023. Growth during the period was attributed to increased awareness regarding gastrointestinal diseases and rising demand for point-of-care testing.
Developments in diagnostic technologies like PCR-based molecular tests were key in driving demand. Diarrheal infections were one of the leading causes of morbidity globally. For example,
The forecast period is predicted to witness continuous developments in molecular diagnostic and automation. Increasing awareness regarding gastrointestinal diseases and their complications are likely to drive demand for innovative diagnostic methods and treatment options.
Increasing Prevalence of Gastrointestinal Infections
The World Health Organization (WHO) estimates that 600 million people fall ill each year due to contaminated food, resulting in 420,000 deaths annually. Rapid urbanization, especially in developing countries, has led to overcrowded living conditions and strained sanitation systems, increasing the risk of GI infections.
Changes in temperature and rainfall patterns have facilitated the spread of GI pathogens, especially waterborne diseases like cholera and typhoid. For instance, Vibrio infections, including cholera, have become more frequent in coastal areas due to rising sea temperatures. For example,
Emergence of antibiotic-resistant bacteria has made some GI infections harder to treat, increasing the demand for accurate diagnostic tools to guide treatment. Pathogens like Clostridioides difficile have become a significant concern in hospital settings.
Surging Adoption of Molecular Diagnostic Technologies
Molecular diagnostic techniques, such as polymerase chain reaction (PCR) and nucleic acid amplification tests (NAATs), can detect pathogens at very low concentrations. Modern molecular diagnostics can simultaneously test for multiple pathogens in a single assay. For instance,
Molecular tests typically provide results within 1 to 4 hours, compared to 24 to 72 hours for culture-based methods. This speed is particularly critical in acute GI infections, where timely diagnosis can prevent complications such as dehydration or sepsis. Automated molecular diagnostic platforms like Cepheid's GeneXpert and Roche's cobas Liat System have made these technologies accessible even in smaller healthcare settings. For instance,
Inadequate Diagnostic Infrastructure in Developing Regions
Healthcare facilities in developing regions lack the necessary infrastructure for conducting advanced diagnostic tests, like polymerase chain reaction (PCR) or multiplex pathogen detection panels. For example,
Unique diagnostic technologies require skilled technicians to operate equipment and interpret results. A 2021 study by the International Journal of Infectious Diseases reported that 70% of laboratories in rural Asia and Africa operate with underqualified staff, leading to lower test accuracy.
Innovations in Diagnostic Technologies
Technologies like polymerase chain reaction (PCR), nucleic acid amplification tests (NAATs), and isothermal amplification have revolutionized pathogen detection. For example,
Portable diagnostic devices like Cepheid GeneXpert and Abbott ID NOW provide results within 30 minutes to 1 hour, enabling immediate clinical decisions. NGS facilitates detailed pathogen profiling, including strain typing, resistance gene identification, and microbiome analysis.
Microfluidic platforms integrate multiple diagnostic processes, including sample preparation and pathogen detection in a single chip. These systems decrease testing time, cost, and required sample volume, making them ideal for low-resource settings.
Rise of Syndromic Testing Panels
Syndromic panels detect numerous pathogens in a single test, addressing the challenge of overlapping symptoms caused by various GI infections. Early and accurate detection of pathogens facilitates prompt treatment, reducing patient morbidity and the risk of complications. For instance,
Syndromic panels streamline diagnostic workflows by combining multiple tests into one, minimizing the need for repeated testing. As per data from the American Society of Microbiology, laboratories report a 40% decrease in workload when switching to multiplex syndromic panels.
Companies in the gastrointestinal infection testing market are investing in molecular diagnostic technologies like polymerase chain reaction and next-generation sequencing, to improve the accuracy and speed of GI infection testing. For instance, BioFire Diagnostics offers the BioFire FilmArray GI Panel, which detects 22 pathogens within 1 hour.
Automated platforms assist in decreasing manual errors and improve workflow efficiency. This is particularly essential for high-throughput laboratories. Businesses are focusing on developing rapid diagnostic kits that provide results within minutes, thereby enabling timely treatment.
Companies are progressively partnering with healthcare providers to promote the adoption of advanced diagnostic technologies. Joint research initiatives with academic institutions enable the development of innovative testing methods.
Recent Industry Developments
|
Attributes |
Detail |
|
Forecast Period |
2025 to 2032 |
|
Historical Data Available for |
2019 to 2023 |
|
Market Analysis |
US$ Billion for Value |
|
Key Regions Covered |
|
|
Key Market Segments Covered |
|
|
Key Companies Profiled in the Report |
|
|
Report Coverage |
|
|
Customization and Pricing |
Available upon request |
By Product Type
By Test Type
By Infection Type
By Sample Type
By End User
By Region
To know more about delivery timeline for this report Contact Sales

The market is anticipated to reach a value of US$ 617.7 Mn by 2032.
Tests for GI include molecular tests, immunoassays, and culture tests.
North America is anticipated to emerge as the leading region with a share of 35% in 2025.
Prominent players in the market include Bio-Rad Laboratories, Inc., Thermo Fisher Scientific, Inc., and Abbott Laboratories.
The market is predicted to witness a CAGR of 5.2% throughout the forecast period.