Industry: Healthcare
Published Date: March-2025
Format: PPT*, PDF, EXCEL
Delivery Timelines: Contact Sales
Number of Pages: 275
Report ID: PMRREP29257
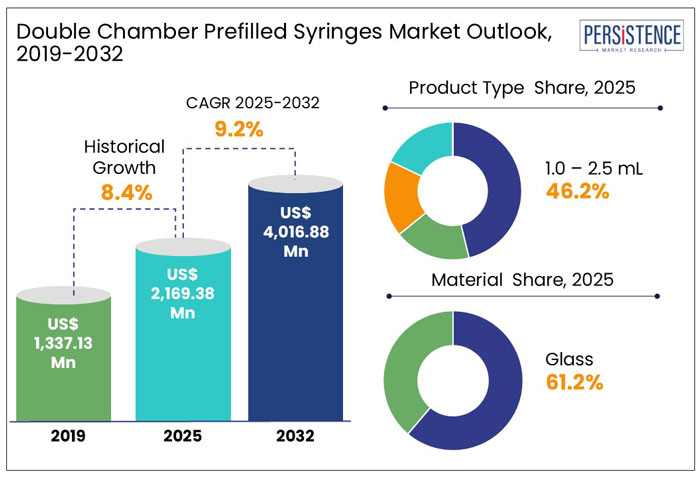
The global double chamber prefilled syringes market is experiencing robust growth, driven by the increasing demand for efficient drug delivery systems, particularly for biologics and medications requiring reconstitution. The market is projected to reach US$ 2,169.4 million in 2025, expanding at a CAGR of 9.2%, and is expected to achieve US$ 4,016.9 million by 2032.
A key driver of this growth is the development and approval of combination products that integrate both drug and device components, enhancing patient convenience and compliance.
Regulatory agencies like the European Medicines Agency (EMA) play a vital role in shaping the double-chamber prefilled syringes market by providing procedural guidance for post-authorization changes, such as the introduction of new pack sizes. These regulations ensure product safety, efficacy, and consistency, fostering innovation in drug delivery systems.
A key example of this innovation is seen in dual-chamber prefilled syringes like Rebinyn® (Coagulation Factor IX) and Tretten® (Coagulation Factor XIII A-Subunit), which are designed for hemophilia treatment. These syringes maintain drug stability and allow for convenient reconstitution, addressing the critical needs of biologics and complex injectable therapies.
Key Highlights of the Double chamber prefilled syringes Industry
|
Global Market Attributes |
Key Insights |
|
Double chamber prefilled syringes Market Size (2025E) |
US$ 2,169.4 Mn |
|
Market Value Forecast (2032F) |
US$ 4,016.9 Mn |
|
Projected Growth (CAGR 2025 to 2032) |
9.2% |
|
Historical Market Growth (CAGR 2019 to 2024) |
8.4% |
The global double chamber prefilled syringes market has grown steadily, with a historical CAGR of 8.4%, driven by the increasing demand for safe and efficient drug delivery systems, particularly for biologics and reconstitution medications.
Key factors include rising chronic disease prevalence, advancements in dual chamber technology, and the convenience of self-administration therapies. Innovative products like XYNTHA® SOLOFUSE, Rebinyn®, and Genotropin® highlight the benefits of maintaining drug stability and reducing contamination risks.
The market is expected to grow at a CAGR of 9.2% from 2025 to 2032, supported by technological advancements, regulatory support for combination products, and a focus on patient-centric solutions. These trends position the market for strong growth in both clinical and home care settings.
Rising Biopharmaceutical Market Boosting Demand for Double Chamber Prefilled Syringes
The rapid expansion of the biopharmaceutical market and the increasing demand for self-administration are major drivers of the double chamber prefilled syringes market. With 98 biologic-device combination products approved by the FDA between 2000 and 2023, the need for safe, precise, and user-friendly drug delivery systems has grown significantly. Dual-chamber prefilled syringes are particularly beneficial for lyophilized biologics, ensuring drug stability, accurate reconstitution, and reduced contamination risks.
Further supporting market growth, regulatory approvals for biologic and biosimilar drugs have surged.
As biologics become more complex and self-administration continues to rise, the demand for advanced prefilled syringe technologies will drive market expansion, making double chamber prefilled syringes a preferred choice for biologic drug delivery.
High Manufacturing Costs and Design Complexity Limit Market Growth
The high manufacturing costs and design complexity of double chamber prefilled syringes pose a significant restraint to market growth. These syringes require advanced manufacturing processes, including precision engineering, specialized materials, and stringent quality control to ensure drug stability and safety. The dual chamber design, which keeps the liquid and lyophilized drug separate until administration, demands sophisticated filling, sealing, and assembly techniques.
For example, Becton, Dickinson and Company's (BD) Physioject™ dual chamber prefilled syringes involve complex manufacturing steps to maintain drug stability and enable easy reconstitution before use. These complexities drive up production costs and necessitate substantial investment in technology and equipment. As a result, the high cost of these advanced syringes can hinder their adoption, especially in cost-sensitive markets. Manufacturers need to focus on balancing innovation with affordability to expand the accessibility of double chamber prefilled syringes globally.
Advanced Reconstitution Technologies Open New Avenues in Double chamber prefilled syringes
Technological advancements in reconstitution devices are creating significant opportunities in the double chamber prefilled syringes market. Innovations like dual-chamber prefilled syringes, vial adapters, and automated reconstitution systems simplify drug preparation, reducing contamination risks and human errors.
Automated reconstitution systems, like Enable Injections' Automatic Reconstitution Transfer System, further streamline drug preparation by minimizing manual steps, improving safety, and enhancing ease of use.
Additionally, needle-free technologies reduce needle-stick injuries and improve patient compliance. As biologic therapies continue to grow, these innovations will drive market expansion by providing safer and more effective drug delivery solutions.
Ensuring Drug Efficacy with Liquid/Powder Dual Chamber Syringes
The Liquid/Powder application in the double-chamber prefilled syringes market is experiencing significant growth and is projected to account for 40.3% of the market share in 2025. This growth is primarily driven by the need to maintain drug stability, particularly for biologics and other sensitive medications that require reconstitution before administration.
The success of such formulations underscores the increasing adoption of Liquid/Powder dual-chamber prefilled syringes in the pharmaceutical industry.
Glass Leads Double Chamber Syringes Market
Glass remains the preferred material for double-chamber prefilled syringes, driven by its superior barrier properties, exceptional chemical stability, and strong regulatory acceptance. Unlike plastic, which is susceptible to drug interactions, permeability issues, and the presence of extractables and leachables (E&L), glass offers an inert and non-reactive surface, ensuring the long-term stability and efficacy of pharmaceuticals.
As a result, the glass segment is projected to dominate the double-chamber prefilled syringes market, accounting for approximately 61.2% of the market share in 2025.
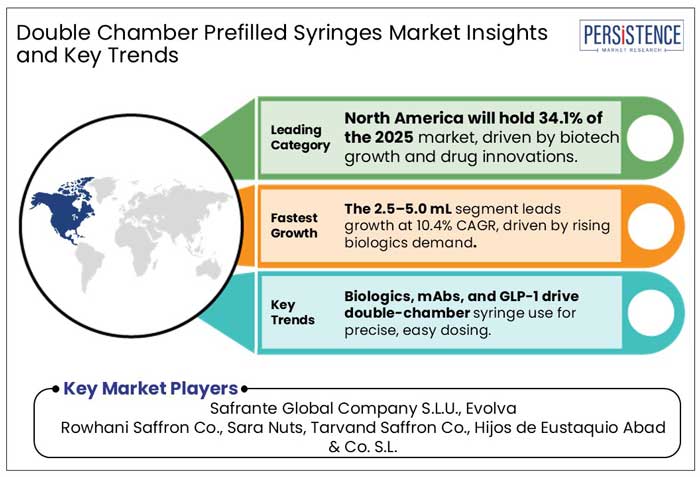
Expanding Biopharma Industry Positions North America as a Key Prefilled Syringe Market
North America is set to account for 34.1% of the global double-chamber prefilled syringes market in 2025, driven by a strong biopharmaceutical industry, rising demand for biologics, and increasing investment in advanced drug delivery systems. The increasing demand for innovative drug delivery solutions, particularly for mRNA-based therapies and GLP-1 medications, is driving investments in syringe manufacturing. For example, SCHOTT Pharma expanded its U.S. production capacity to meet this growing need, strengthening North America's position in the double-chamber prefilled syringes market.
Rising Demand for Biologics Fuels Asia Pacific Market Expansion
The Asia Pacific region is projected to achieve a 10.2% CAGR in 2025, driven by the rapid growth of the biopharmaceutical industry, increasing demand for biologics and self-administrable drug delivery systems, and advancements in healthcare infrastructure. Additionally, rising investments in pharmaceutical manufacturing, supportive government policies, and the growing prevalence of chronic diseases are fueling the adoption of double-chamber prefilled syringes in the region.
The double chamber prefilled syringes market is competitive, with key players like BD, Nipro Corporation, Gerresheimer AG, and SCHOTT AG focusing on innovation, advanced syringe designs, and strategic partnerships. Companies are enhancing manufacturing capacities and aligning with pharmaceutical firms to meet the rising demand for biologics and complex drug formulations. Partnerships with pharmaceutical companies and regulatory compliance are critical strategies to gain a competitive edge.
|
Report Attributes |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis Units |
Value: US$ Mn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Product Type
By Material
By Application
By Distribution Channel
By Region
To know more about delivery timeline for this report Contact Sales

The global Double chamber prefilled syringes market is estimated to increase from US$ 2,169.4Mn in 2025 to US$ 4,016.9 Mn in 2032.
The global double chamber prefilled syringes market is propelled by the growing demand for efficient drug delivery systems, particularly for biologics and medications requiring reconstitution, along with advancements in syringe technology and patient-centric healthcare solutions.
The market is projected to record a CAGR of 9.2% during the forecast period from 2025 to 2032.
Major players include BD, NIPRO PHARMA CORPORATION, SCHOTT Pharma, Gerresheimer AG, among others.
Opportunities in the double chamber prefilled syringes market include increasing demand for biologics and injectable therapies, growth in self-administration treatments, advancements in dual chamber technology, and expanding applications in emerging markets with a focus on improved drug stability, safety, and patient convenience.