Industry: Healthcare
Published Date: April-2025
Format: PPT*, PDF, EXCEL
Delivery Timelines: Contact Sales
Number of Pages: 180
Report ID: PMRREP17032
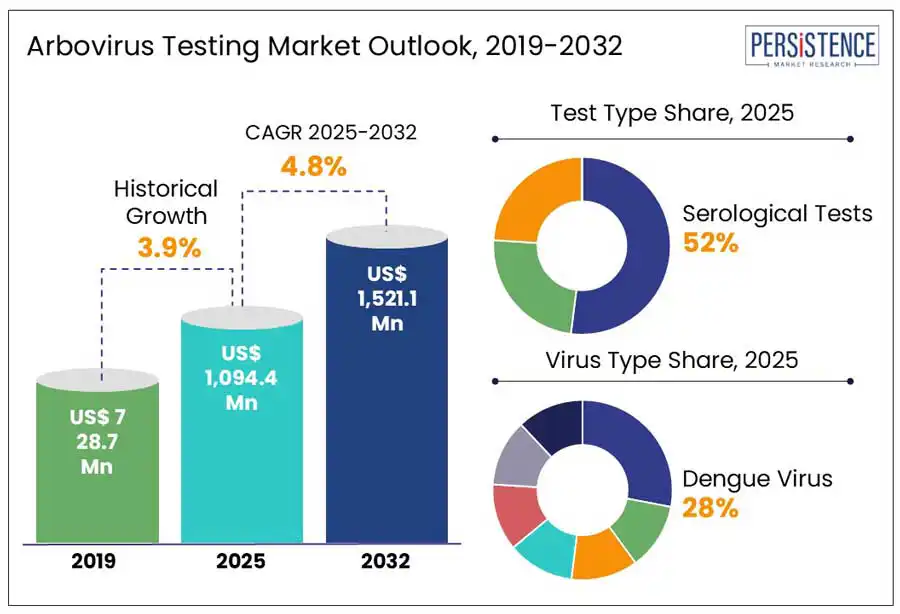
The global arbovirus testing market is projected to witness a CAGR of 4.8% from 2025 to 2032. It is anticipated to increase from US$ 1,094.4 Mn recorded in 2025 to a staggering US$ 1,521.1 Mn by 2032. The market is experiencing steady growth due to the rising incidence of arboviral infections such as dengue, Zika, chikungunya, and yellow fever, particularly in tropical and sub-tropical regions. Increased global travel, urbanization, and climate change have expanded the geographic reach of these viruses, driving demand for effective diagnostic tools. Technological advancements in molecular diagnostics, such as RT-PCR and ELISA have improved the accuracy and speed of arbovirus detection.
Key Industry Highlights:
|
Global Market Attribute |
Key Insights |
|
Arbovirus Testing Market Size (2025E) |
US$ 1,094.4 Mn |
|
Market Value Forecast (2032F) |
US$ 1,521.1 Mn |
|
Projected Growth (CAGR 2025 to 2032) |
4.8% |
|
Historical Market Growth (CAGR 2019 to 2024) |
3.9% |
The growing impact of climate change on vector ecology. Rising global temperatures, shifting rainfall patterns, and increased humidity are fundamentally altering the geographical distribution of disease-carrying mosquitoes, particularly Aedes aegypti and Aedes albopictus. This environmental shift is not gradual, but accelerating with global warming, thus shortening mosquito incubation periods and long transmission seasons. Additionally, extreme weather events such as floods and hurricanes create ideal conditions for mosquito breeding, often overwhelming public health systems. The sudden emergence of arboviruses in new geographies is creating an urgent need for expanded surveillance and rapid, and accurate diagnostic testing.
The high degree of cross-reactivity in serological testing, particularly among closely related viruses such as dengue, zika, and chikungunya challenges the course of treatment. These viruses often co-circulate in endemic regions and share structural similarities in their envelope proteins, which makes it difficult for standard serological assays, especially those based on IgM or IgG antibodies to differentiate between them accurately. As a result, a patient previously exposed to dengue may test positive for zika antibodies, despite never being infected with zika, and vice-versa. This leads to false positives or ambiguous results, complicating clinical decision-making, especially in cases of expecting mothers who are at the risk of congenital zika syndrome.
By forming strategic alliances with travel clinics, embassies, visa centers, and international health hubs, service providers can offer customized arbovirus screening packages. These packages include rapid testing, serology panels, travel-specific health consultations, and follow-up care, all aligned with the traveler’s destination and risk level. This service is essential for high-risk travelers such as expecting mothers, geriatric, or immunocompromised. On the return side, post-travel screening helps detect asymptomatic carriers and prevent local transmission, which is especially important in regions where vectors are present but the virus is not yet endemic. Providers can also integrate this service with travel insurance or loyalty programs, making it both accessible and incentivized.
Serological tests dominate the arbovirus testing market due to their unique ability to balance accessibility, scalability, and diagnostic utility in both outbreak and surveillance contexts. Serological methods such as ELISA and rapid diagnostic tests (RDTs) are cost-effective, portable, and simple to use, making them ideal for deployment in resource-limited, high-risk regions where arboviral infections such as dengue or Zika are endemic. These tests are crucial for identifying both current and past infections through antibody detection, which is vital for understanding population exposure and immunity trends.
The dengue virus segment is expected to lead due to its widespread prevalence and significant public health impact. According to the World Health Organization (WHO), affecting over 390 million people annually, dengue has become an endemic in more than 100 countries, particularly in tropical and sub-tropical regions such as Asia Pacific and Latin America. Frequent outbreaks and seasonal surges drive a continuous demand for rapid and accurate diagnostic tools. Governments and international health organizations have prioritized dengue surveillance and control, leading to increased funding and testing programs.
Hospitals and clinics dominate the end-user segment in the arbovirus testing market due to their central role in frontline healthcare delivery, especially during outbreaks. These facilities are often the first point of contact for symptomatic patients enabling early diagnosis and immediate intervention. Their access to a broad range of diagnostic tools from rapid tests to advanced molecular assays ensures timely and accurate results. Additionally, hospitals and clinics often operate under government surveillance programs for notifiable diseases such as dengue and Zika making them pivotal in outbreak detection and reporting.
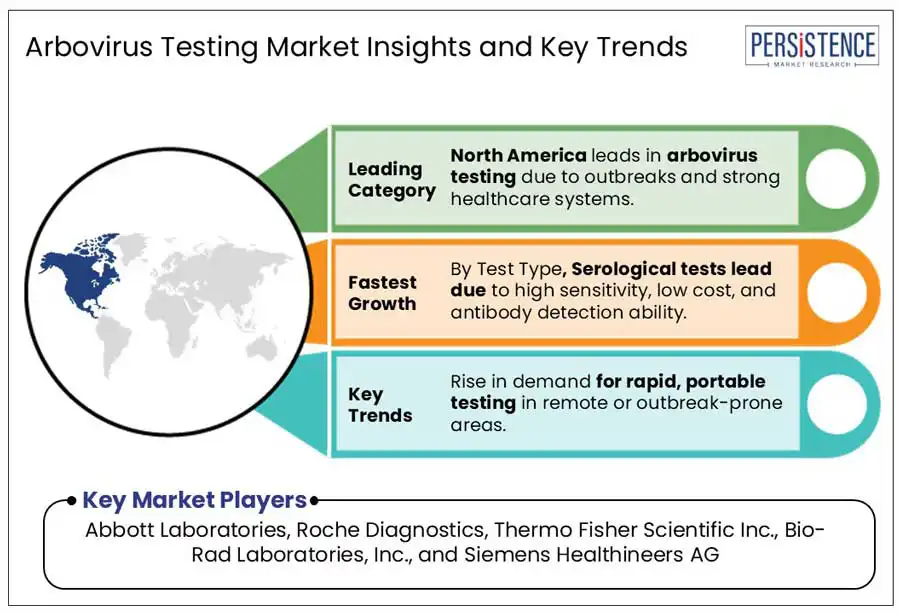
The U.S. arbovirus testing market is witnessing steady growth due to its sophisticated healthcare system, extensive surveillance networks (such as those operated by the CDC), and strong R&D ecosystem. The country faces seasonal and regional outbreaks of West Nile virus and implemented strict blood screening regulations to prevent transmission. Government-backed initiatives such as mosquito control programs and disease reporting systems support early detection and containment of arboviral outbreaks.
Latin America has emerged as a critical region in the arbovirus testing market due to its persistent exposure to endemic and epidemic arboviral diseases such as dengue, Zika, chikungunya, and yellow fever. The region's tropical climate, high urban population density, and widespread Aedes mosquito presence creates ideal conditions for transmission. Countries such as Brazil, Colombia, and Mexico face frequent outbreaks, prompting governments to invest heavily in surveillance, rapid diagnostics, and public health infrastructure.
Rapid urbanization, climate change, and expanding mosquito habitats have contributed to frequent arboviral outbreaks, prompting governments to invest heavily in public health initiatives and disease surveillance programs. Countries like India, China, Thailand, and Indonesia are particularly affected and have ramped up efforts in early diagnosis and containment. Technological advancements in molecular diagnostics and the growing availability of point-of-care testing kits are transforming disease detection in remote and underserved areas.
The global arbovirus testing market is highly fragmented. Companies invest in Research & Development for rapid, accurate, and cost-effective diagnostic solutions. Collaborations with public health agencies and international organizations further enhance their market position. The landscape is shaped by increase in government support, rising outbreak incidences, and growing demand for point-of-care testing in endemic regions.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis Units |
Value: US$ Mn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Test Type
By Virus Type
By End-user
By Region
To know more about delivery timeline for this report Contact Sales

The global arbovirus testing market is estimated to increase from US$ 1,094.4 Mn in 2025 to US$ 1,521.1 Mn in 2032.
Increasing outbreaks of diseases such as Dengue, Zika, Chikungunya, and West Nile virus especially in tropical and sub-tropical regions are driving demand for timely and accurate diagnostic solutions.
The market is projected to record a CAGR of 4.8% during the forecast period from 2025 to 2032.
Tele-dermatology and mobile health platforms present new avenues for diagnosis, prescription, and patient monitoring.
The key players in the arbovirus testing market include Abbott Laboratories, Roche Diagnostics, Thermo Fisher Scientific Inc., Bio-Rad Laboratories, Inc., and Siemens Healthineers AG, and others.