Comprehensive Snapshot of Antimicrobial Resistance Diagnostics Market Report Including Regional and Country Analysis in Brief.
Industry: Healthcare
Published Date: April-2025
Format: PPT*, PDF, EXCEL
Delivery Timelines: Contact Sales
Number of Pages: 160
Report ID: PMRREP35211
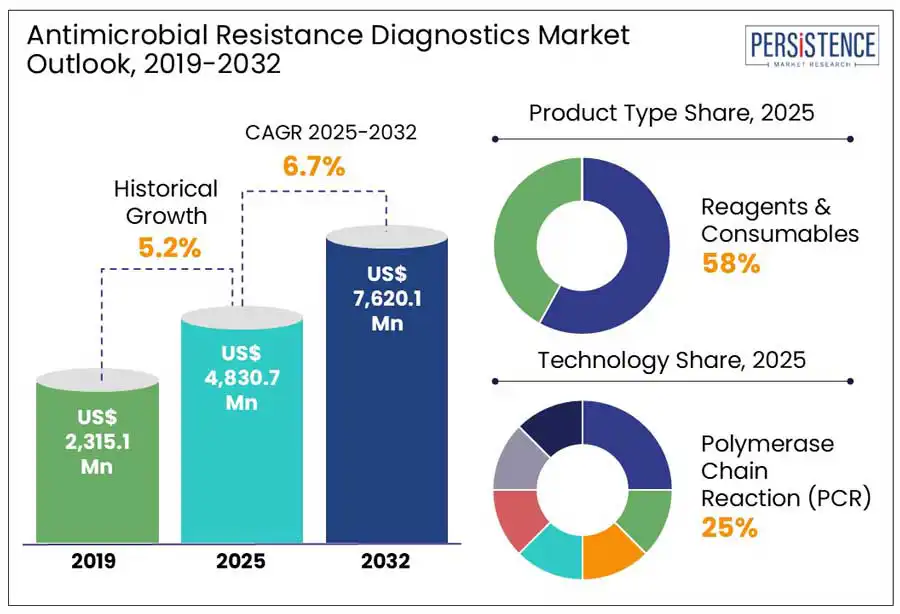
The global antimicrobial resistance diagnostics market size is projected to witness a CAGR of 6.7% from 2025 to 2032. It is anticipated to increase from US$4,830.7 Mn recorded in 2025 to a staggering US$ 7,620.1 Mn by 2032.
The antimicrobial resistance (AMR) diagnostics market is experiencing significant growth due to the rising prevalence of drug-resistant infections and the urgent need for rapid, accurate detection methods. Advances in molecular diagnostics, such as PCR and next-generation sequencing (NGS), are driving market expansion. Government initiatives, increased funding for AMR research, and growing awareness among healthcare professionals further support market growth.
Key Industry Highlights:
|
Global Market Attribute |
Key Insights |
|
Antimicrobial Resistance Diagnostics Market Size (2025E) |
US$ 4,830.7 Mn |
|
Market Value Forecast (2032F) |
US$ 7,620.1 Mn |
|
Projected Growth (CAGR 2025 to 2032) |
6.7% |
|
Historical Market Growth (CAGR 2019 to 2024) |
5.2% |
The increasing prevalence of multidrug-resistant (MDR) bacteria, such as carbapenem-resistant Enterobacteriaceae (CRE), methicillin-resistant Staphylococcus aureus (MRSA), and extensively drug-resistant (XDR) tuberculosis, is severely limiting treatment options. This has prompted governments, healthcare agencies, and regulatory bodies to push for advanced AMR diagnostic solutions that enable early detection and targeted therapy, reducing unnecessary antibiotic use and improving patient outcomes.
Healthcare systems worldwide are implementing national AMR surveillance programs, such as the Global Antimicrobial Resistance and Use Surveillance System (GLASS) by WHO to track resistance patterns and enhance preparedness. Additionally, rapid molecular diagnostics, including PCR-based assays, next-generation sequencing (NGS), and biosensor-based AMR detection, are gaining traction due to their ability to quickly identify resistant pathogens, leading to better infection control and antimicrobial stewardship.
With the World Health Organization (WHO) estimating that AMR could cause 10 million deaths annually by 2050, the urgency to combat this crisis drives heavy investments in innovative AMR diagnostics, making the market a key player against drug-resistant infections.
A key challenge in the antimicrobial resistance diagnostics market is the limited availability of effective treatment options once resistance is detected. While advanced AMR diagnostics can quickly identify resistant pathogens the lack of new antibiotics severely limits treatment choices, reducing the clinical impact of diagnostics. Physicians often find that even when AMR is early detected, the absence of effective second or third-line therapies makes the diagnostic result less actionable. This leads to a reluctance to invest in rapid AMR tests, especially in hospital settings where empirical antibiotic use remains prevalent.
Furthermore, antibiotic stewardship programs, while crucial for combating resistance, often restrict the use of last-resort antibiotics, further complicating treatment decisions. Without parallel advancements in novel antimicrobial development, the demand for AMR diagnostics remains constrained, as physicians see limited value in detecting resistance without viable treatment alternatives. This creates a market bottleneck, hindering widespread adoption.
The industry is driven by technological innovation, rising global awareness, and the urgent need to combat antibiotic resistance. A key highlight includes Sysmex Astrego’s award-winning rapid test for urinary tract infections, delivering results in under an hour down from two to three days, helping reduce inappropriate antibiotic use. Major opportunities lie in point-of-care testing (POCT), integration of AI-driven bioinformatics platforms, and strategic acquisitions by companies like Roche and Hologic. The future of AMR diagnostics hinges on expanding access to rapid, affordable solutions and leveraging digital tools for improved antimicrobial stewardship
The reagents & consumables dominates due to its recurring demand and essential role in testing workflows. Diagnostic instruments, which are one-time capital investments, reagents and consumables such as assay kits, culture media, and antimicrobial susceptibility testing (AST) panels must be replenished regularly, ensuring sustained market growth. The rising adoption of rapid molecular diagnostics, including PCR-based and immunoassay-based AMR detection, has further boosted the need for specialized reagents. Additionally, the expansion of AMR surveillance programs by global organizations like the WHO and Centers for Disease Control and Prevention (CDC) has increased the demand for standardized consumables used in routine screening. With hospitals and diagnostic labs prioritizing rapid, accurate, and cost-effective AMR testing, the consumables segment benefits from continuous purchasing cycles.
Polymerase Chain Reaction (PCR) dominates the antimicrobial resistance diagnostics market owing to its high sensitivity, rapid turnaround time, and ability to detect resistance genes directly from clinical samples. Unlike traditional microbiology culture methods, which takes 24 to 72 hours to yield results, PCR can identify resistant pathogens in a few hours, enabling quicker clinical decision-making. This speed is critical in combating multidrug-resistant (MDR) infections, where delays in diagnosis can worsen patient outcomes.
PCR’s ability to detect genetic resistance markers before phenotypic expression makes it superior for early intervention, reducing reliance on empirical antibiotic therapy. Moreover, multiplex PCR panels allow simultaneous detection of multiple resistance genes, increasing efficiency in hospital settings. Advancements such as real-time PCR (qPCR) and digital PCR (dPCR) further enhance accuracy by minimizing false negatives.
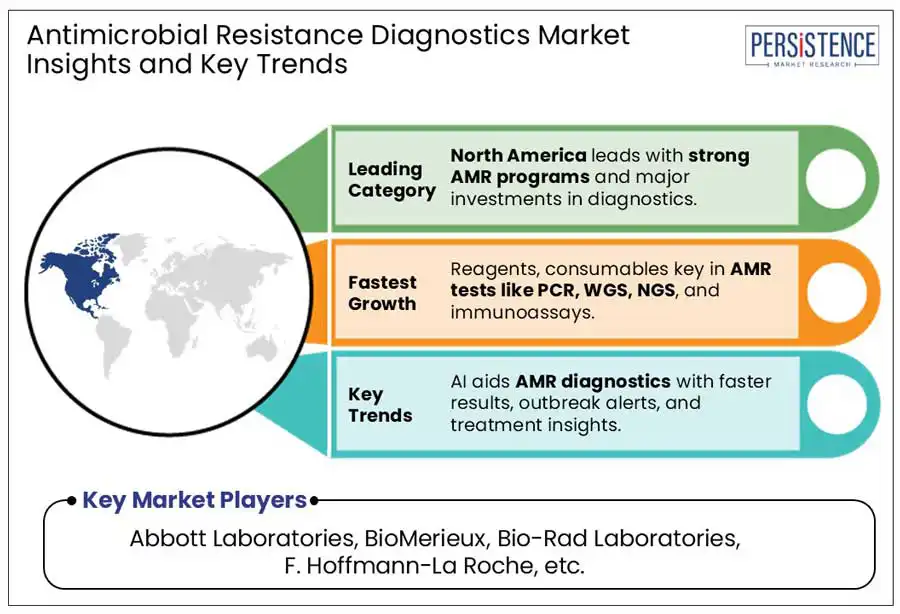
North America is projected to lead in 2025 due to its advanced healthcare infrastructure, strong regulatory support, and high adoption of molecular diagnostics. The region benefits from significant investments in R&D, biotechnology, and infectious disease surveillance programs, enabling faster integration of cutting-edge diagnostic technologies such as PCR, Next-Generation Sequencing (NGS), and mass spectrometry. Additionally, the U.S. is at the forefront of Point-of-Care (PoC) diagnostic adoption, particularly in antimicrobial resistance (AMR) detection. With the rising concerns over hospital-acquired infections (HAIs) and antibiotic-resistant pathogens, healthcare facilities are increasingly integrating rapid AMR detection technologies to enhance patient outcomes. Hospitals, urgent care centers, and outpatient clinics are leveraging molecular diagnostic tools, microfluidic platforms, and biosensors to deliver faster and more accurate results.
Due to its strong regulatory enforcement, cross-border collaboration, and increasing hospital surveillance programs, Europe is poised to witness a fast growth in upcoming years. The European Union (EU) mandates stringent AMR monitoring, requiring healthcare facilities to implement routine resistance testing, particularly in high-risk environments like ICUs and long-term care units. Organizations such as the European Medicines Agency (EMA) and the European Centre for Disease Prevention and Control (ECDC) actively track AMR trends, encouraging the widespread adoption of advanced diagnostics.
The region’s growing burden of infectious diseases, such as tuberculosis (TB) and multidrug-resistant bacterial infections has led to an urgent need for rapid, cost-effective AMR detection tools. Asia Pacific has a high demand for low-cost, portable diagnostic solutions that functions in resource-limited rural healthcare settings. This has driven the adoption of affordable point-of-care (PoC) diagnostics and lab-on-a-chip technologies.
International collaborations with pharmaceutical and biotech firms are increasing, leading to technology transfers and the localization of advanced AMR diagnostic manufacturing. Governments in countries like Saudi Arabia, South Africa, and the UAE are also strengthening regulatory frameworks, promoting incentives for diagnostic innovations. Another emerging opportunity is the integration of artificial intelligence (AI) and genomic sequencing in AMR surveillance, enabling predictive analytics for outbreak control. Furthermore, the region’s rising adoption of telemedicine and digital health platforms creates a pathway for AI-powered AMR diagnostic tools that can be deployed in remote areas.
The global antimicrobial resistance diagnostics market is fragmented. Industry leaders such as bioMérieux, BD (Becton, Dickinson and Company), Roche, Thermo Fisher Scientific, and Abbott dominate the AMR diagnostics space with a strong portfolio of molecular and culture-based solutions. These companies are leveraging AI-driven diagnostic tools and expanding automation in AMR testing to improve turnaround times.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis Units |
Value: US$ Mn, Volume as applicable |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Product Type
By Technology
By Pathogen Type
By End-user
By Region
To know more about delivery timeline for this report Contact Sales

The global antimicrobial resistance diagnostics market is estimated to increase from US$ 4,830.7 Mn in 2025 to US$ 7,620.1 Mn in 2032.
Innovations in PCR-based testing, whole-genome sequencing, next-generation sequencing (NGS), and AI-driven diagnostic tools are enabling faster and more precise AMR detection, reducing reliance on traditional culture-based methods.
The market is projected to record a CAGR of 6.7% during the forecast period from 2025 to 2032.
The growing demand for portable, real-time diagnostics in hospitals, clinics, and remote settings presents significant opportunities for companies developing rapid AMR detection tools.
The key players in the Antimicrobial Resistance Diagnostics Market include Abbott Laboratories, Accelerated Diagnostics, USA, Alifax, Bio-Rad Laboratories, BioMerieux, Beckman Coulter, and others.