Industry: Healthcare
Published Date: August-2024
Format: PPT*, PDF, EXCEL
Delivery Timelines: Contact Sales
Number of Pages: 190
Report ID: PMRREP34626
The global market for allogeneic cell therapy is estimated to value at US$2.4 Bn by the end of 2031 from US$0.4 Bn recorded in 2024. the market is expected to secure a CAGR of 24.1% in the forthcoming years from 2024 to 2031.
Key Highlights of the Market
|
Market Attributes |
Key Insights |
|
Market Size (2024E) |
US$0.4 Bn |
|
Projected Market Value (2031F) |
US$2.4 Bn |
|
Forecast Growth Rate (CAGR 2024 to 2031) |
24.1% |
|
Historical Growth Rate (CAGR 2018 to 2023) |
23.4% |
The market for Allogeneic Cell Therapy is a rapidly expanding segment within the broader regenerative medicine and advanced therapeutics field. Allogeneic cell therapy involves using cells derived from a donor to treat various diseases and medical conditions in different patients.
This approach contrasts with autologous cell therapy, where a patient's cells are used. The main advantage of allogeneic therapies lies in their scalability and potential for mass production, enabling the treatment of numerous patients from a single donor source.
Several key factors, including advancements in stem cell research and biotechnology, increasing prevalence of chronic and degenerative diseases, and growing investments from both public and private sectors drive the market for allogeneic cell therapies.
Innovations in cell engineering, improved understanding of cellular mechanisms, and advancements in manufacturing processes have significantly boosted the development of allogeneic cell therapies.
Notably, regulatory agencies are playing a crucial role in supporting the clinical development and approval of these therapies, thereby enhancing their credibility and potential to address unmet medical needs.
The allogeneic cell therapy market is evolving with innovations like in-vivo cell expansion strategies, aiming to simplify treatment processes. Researchers are also developing hypoallergenic or haploidentical stem cells to expand donor compatibility and therapeutic options.
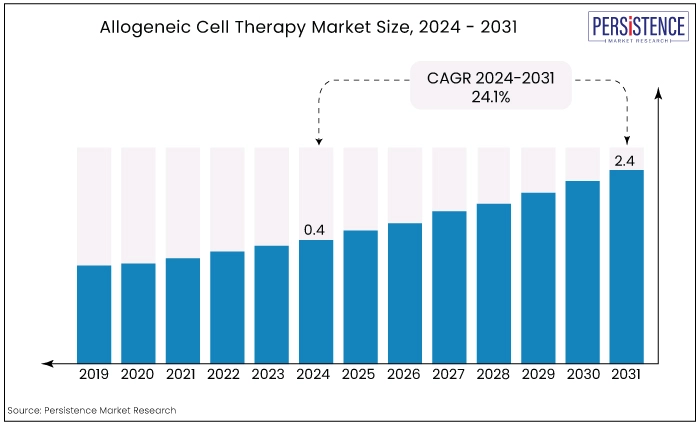
The allogeneic cell therapy market has transitioned from foundational research to advanced clinical applications. Historically, growth was gradual, focused on hematopoietic stem cell transplants for blood disorders.
Recently, the market has surged due to technological advancements, increased funding, and successful clinical trials showcasing efficacy in treating various conditions.
Currently, the market is expanding rapidly, driven by improved cell engineering techniques, scalable manufacturing processes, and supportive regulatory environments. Investments from biotech firms and pharmaceutical companies are further propelling growth.
Prospects are promising, with anticipated breakthroughs in personalized cell therapies and wider applications in autoimmune diseases, genetic disorders, and tissue regeneration. As research advances and new treatments emerge, allogeneic cell therapy is set to revolutionize regenerative medicine and significantly enhance patient care.
Increased Demand for Healthy Allogeneic Donors and Advancements in Regenerative Medicine
The increased demand for healthy allogeneic donors is driven by advancements in regenerative medicine, which require a reliable and diverse pool of donor cells for therapeutic applications.
As the field progresses, there is a growing emphasis on sourcing cells from healthy donors to ensure optimal efficacy and safety in treatments. This demand underscores the importance of stringent donor screening protocols and ethical considerations in selecting suitable donors.
Advancements in regenerative medicine, such as improved cell isolation techniques, genetic engineering, and understanding of immunological compatibility, are enhancing the viability and effectiveness of allogeneic cell therapies.
Such innovations are expanding the scope of treatments available, ranging from hematological disorders to complex tissue regeneration scenarios.
As research continues to refine these technologies, the integration of healthy donor cells in regenerative medicine holds promise for addressing previously untreatable conditions and improving patient outcomes.
Increasing Incidence of Chronic Diseases
The increasing incidence of chronic diseases is driving growth in the allogeneic cell therapy market, as these therapies offer potential solutions where conventional treatments fall short.
Conditions such as diabetes, cardiovascular diseases, and various cancers are becoming more prevalent globally, necessitating innovative approaches like allogeneic cell therapy.
These therapies harness the potential of donor-derived cells to address underlying disease mechanisms, providing opportunities for disease modification and symptom management.
The rising demand for effective, long-term solutions for chronic illnesses has spurred research and development in cell engineering, immunotherapy, and personalized medicine.
As clinical trials demonstrate promising results and regulatory approvals facilitate market entry, the landscape of allogeneic cell therapy continues to evolve.
The integration of advanced biotechnological techniques with clinical applications holds promise for transforming treatment paradigms and improving quality of life for patients facing chronic diseases.
High Production Costs
High production costs pose a significant challenge in the allogeneic cell therapy market. The complex processes involved in cell sourcing, manufacturing, and quality control contribute to elevated expenses.
The costs stem from stringent regulatory requirements, specialized equipment, and skilled personnel needed for handling and processing donor cells. Additionally, the need for extensive clinical testing and validation further drives up production expenditures.
Addressing these challenges requires innovations in manufacturing technologies, automation, and scalability to reduce costs without compromising product quality or safety.
As advancements continue and economies of scale are achieved, overcoming high production costs will be crucial for expanding accessibility and affordability of allogeneic cell therapies in the healthcare market.
Rising Government Activities for Improved Healthcare Insurance Frameworks
Rising government activities to enhance healthcare insurance frameworks are pivotal for the allogeneic cell therapy market.
Governments worldwide are increasingly recognizing the potential of advanced therapies like allogeneic cell therapy to improve patient outcomes and reduce long-term healthcare costs.
Key initiatives include-
These efforts foster a supportive environment for biotech firms and pharmaceutical companies to invest in and develop allogeneic cell therapies.
Governments can facilitate greater adoption of these therapies by ensuring broader insurance coverage and reducing financial barriers for patients, ultimately driving growth and innovation in the global healthcare landscape.
Advancements in Allogeneic Cell Cancer Immunotherapy
The increasing incidence of chronic diseases is driving growth in the allogeneic cell therapy market, as these therapies offer potential solutions where conventional treatments fall short.
Conditions such as diabetes, cardiovascular diseases, and various cancers are becoming more prevalent globally, necessitating innovative approaches like allogeneic cell therapy.
These therapies harness the potential of donor-derived cells to address underlying disease mechanisms, providing opportunities for disease modification and symptom management.
The rising demand for effective, long-term solutions for chronic illnesses has spurred research and development in cell engineering, immunotherapy, and personalized medicine.
As clinical trials demonstrate promising results and regulatory approvals facilitate market entry, the landscape of allogeneic cell therapy continues to evolve.
The integration of advanced biotechnological techniques with clinical applications holds promise for transforming treatment paradigms and improving quality of life for patients facing chronic diseases.
In-Vivo Cell Expansion Strategies
Researchers are exploring methods to stimulate the expansion of allogeneic cells directly within the patient's body. This approach, if successful, could eliminate the need for complex ex vivo manipulation, leading to potentially faster, more cost-effective, and readily available treatments.
Development of Hypoallergenic or Haploidentical Stem Cells
Research efforts are directed towards creating allogeneic cell therapies derived from either hypoallergenic donor cells with targeted MHC (Major Histocompatibility Complex) disruptions or haploidentical stem cells that have undergone gene editing for single-allele HLA haplotype inactivation. This could significantly broaden the pool of compatible donors for patients in need.
|
Category |
Projected CAGR through 2031 |
|
Source Type - Human |
24.7% |
|
Production Type - In-Vitro |
25.1% |
Stem Cell Therapies Holds a Substantial Market Share
Due to their diverse applications and therapeutic potential, stem cell therapies constitute a significant segment within the allogeneic cell therapy market. These therapies utilize donor-derived stem cells to address various medical conditions, ranging from hematological disorders to tissue regeneration and immune modulation.
The market's growth is driven by advancements in stem cell research, which have enabled the development of novel therapies like induced pluripotent stem cells (iPSCs), and mesenchymal stem cells (MSCs).
Clinical successes and expanding regulatory approvals further bolster their adoption. As research continues to uncover new applications and refine treatment protocols, stem cell therapies are poised to expand their footprint in regenerative medicine, offering hope for patients with complex and previously untreatable conditions.
Dermatological Disorders to Demonstrate the Fastest Growth Rate Through 2031
Dermatological disorders are projected to exhibit the fastest growth rate within the allogeneic cell therapy market over the forecasted period. This growth is driven by rising incidences of conditions like psoriasis, vitiligo, and chronic wounds, which are challenging to treat with conventional therapies.
Allogeneic cell therapies offer promising alternatives by leveraging donor-derived cells to repair damaged tissues, modulate immune responses, and promote skin regeneration.
Advancements in cell engineering and clinical trials are expanding treatment options and enhancing efficacy in dermatological care. Regulatory support and increased investments in research are further accelerating market growth.
As these therapies gain traction, they hold potential to revolutionize dermatology by addressing unmet medical needs and improving the quality of life for patients globally, a testament to our collective care and empathy.
|
Region |
CAGR through 2034 |
|
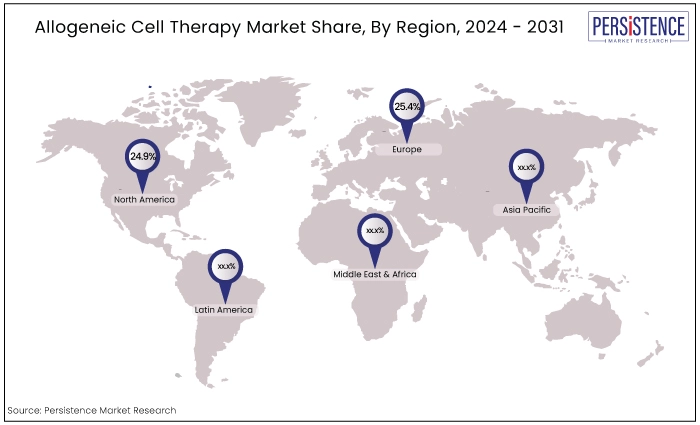
North America |
24.9% |
|
Europe |
25.4% |
North America at the Forefront of Global Market Growth
North America is prominent in the allogeneic cell therapy market, primarily driven by its robust research and development infrastructure, substantial healthcare investments, and a supportive regulatory environment.
The region, spearheaded by the US, hosts leading biotech firms and academic institutions at the forefront of innovation in cell therapy.
Significant advancements in gene editing technologies and scalable production methods have contributed to the market's growth trajectory.
Moreover, the FDA's efficient regulatory pathways, such as accelerated approvals, streamline the path to market for novel therapies, reinforcing North America's pivotal role in the global landscape of allogeneic cell therapies.
Collaborations between industry leaders and research organizations further bolster innovation, solidifying the region's leadership in advancing these transformative treatments worldwide.
Europe Allogeneic Cell Therapy Market Grows Steadily with Strong Research Infrastructure Supporting Advanced Therapies.
Europe's market is experiencing steady growth, bolstered by a robust research infrastructure supporting advanced therapies' development and adoption.
Countries like Germany, the UK, and France lead in research and clinical trials, leveraging initiatives such as the European Medicines Agency's ATMP regulation.
This regulatory framework facilitates the approval and commercialization of innovative cell therapies, enhancing market dynamics. Additionally, collaborations between academic institutions, biotech firms, and pharmaceutical companies drive innovation.
The region's focus on expanding treatment options and improving patient outcomes underscores its pivotal role in the global advancement of allogeneic cell therapies.
The competitive landscape of the monoclonal antibodies market is dominated by key players like Biocon Biologics Ltd, Pfizer, and Biogen Inc. These companies drive innovation through extensive R&D, strategic partnerships, and robust pipelines. Emerging biotechs and regional firms also contribute, fostering a dynamic and evolving market environment.
January 2024
MaxCyte and Wugen have partnered in a strategic platform license agreement to enhance the scale-up of clinical and commercial manufacturing for Wugen's investigational allogeneic cell therapies targeting cancers. This collaboration aims to expedite the development and accessibility of groundbreaking cancer treatments
March 2023
Aurion Biotech has announced regulatory approval from Japan’s Pharmaceuticals and Medical Devices Agency (PMDA) for its innovative cell therapy, Vyznova, intended for treating bullous keratopathy of the cornea.
|
Attributes |
Details |
|
Forecast Period |
2024 to 2031 |
|
Historical Data Available for |
2018 to 2023 |
|
Market Analysis |
US$ Billion for Value |
|
Key Regions Covered |
|
|
Key Market Segments Covered |
|
|
Key Companies Profiled in the Report |
|
|
Report Coverage |
|
|
Customization & Pricing |
Available upon request |
By Therapy Type
By Therapeutic Area
By Region
To know more about delivery timeline for this report Contact Sales

The demand for allogeneic cell therapy is surging due to its potential for off-the-shelf treatments, scalability, and advancements in genetic engineering, enhancing treatment effectiveness and accessibility.
Innovations such as in vivo cell expansion strategies, gene editing technologies, and enhanced manufacturing processes are reshaping the allogeneic cell therapy market, driving its growth and accessibility.
Allogeneic cell therapy is regulated by stringent guidelines ensuring safety, efficacy, and quality, overseen by regulatory bodies like the FDA in the United States and the EMA in Europe.
Future trends in the cell therapy market include advancements in gene editing technologies, expanded use of allogeneic therapies, and innovations in manufacturing processes to enhance scalability and reduce costs.
North America to account for the significant share in the market.