Industry: Healthcare
Published Date: October-2024
Format: PPT*, PDF, EXCEL
Delivery Timelines: Contact Sales
Number of Pages: 179
Report ID: PMRREP34842
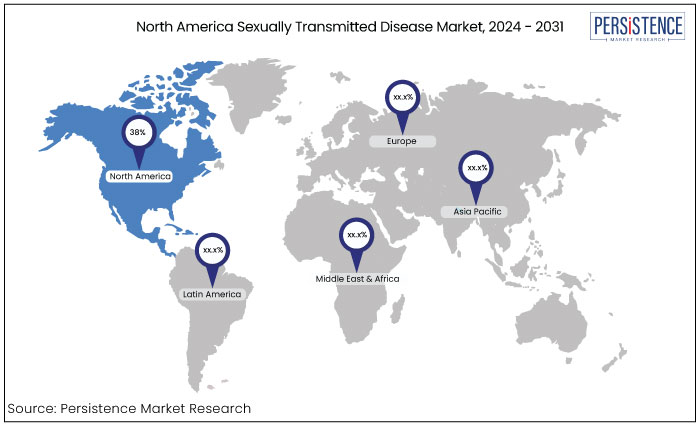
The sexually transmitted disease testing market is estimated to increase from US$10.03 Bn in 2024 to US$17.08 Bn by 2031. The market is projected to record a CAGR of 7.9% during the forecast period from 2024 to 2031. The increased incidence of sexually transmitted diseases such as HIV, chlamydia, gonorrhea, and syphilis globally is driving demand for testing services. North American region dominates the market with 38% of the total market.
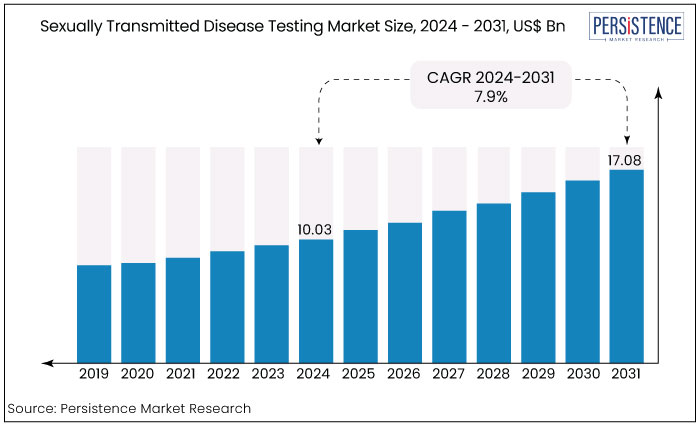
Key Highlights of the Market
|
Market Attributes |
Key Insights |
|
Market Size (2024E) |
US$10.03 Bn |
|
Projected Market Value (2031F) |
US$17.08 Bn |
|
Global Market Growth Rate (CAGR 2024 to 2031) |
7.9% |
|
Historical Market Growth Rate (CAGR 2019 to 2023) |
6.2% |
|
Region |
Market Share in 2024 |
|
North America |
38% |
North America market is estimated to account for a market share of 38% and is projected to sustain its dominance during the forecast period. This pre-eminence can be ascribed to elevated testing rates, proactive governmental initiatives, healthcare infrastructure enhancements, and significant stakeholders' involvement.
Reimbursement policies and advantageous government efforts are expected to propel market expansion. The Centers for Medicare & Medicaid Services (CMS) have introduced the New Technology Add-on Payment (NTAP) program, a significant initiative aimed at mitigating hospital financial losses. The rise in collaborations between in-vitro diagnostic firms and non-profit groups to enhance awareness of STDs generates profitable growth prospects in the STD diagnostics sector.
|
Category |
Market Share in 2024 |
|
Product - Consumables |
70% |
The market for sexually transmitted disease testing is segmented into instruments & services, consumables, and software. Among these, consumables segment dominates the market. The consumables category is estimated to lead the market with a revenue share of 70%. It is projected to see the high CAGR during the forecast period due to rising demand for consumables and the launch of novel solutions.
The rise in product approvals to meet the growing demand for STD diagnostics may facilitate the expansion of related consumables in the market. Abbott's Alinity m STI Assay obtained FDA certification. This is a multiplex assay capable of simultaneously detecting and distinguishing four prevalent sexually transmitted diseases such as Neisseria gonorrhoeae (NG), Chlamydia trachomatis (CT), and Mycoplasma genitalium (MG).
|
Category |
Market Share in 2024 |
|
Technology - Molecular Diagnostics |
45% |
The market is divided into immunoassay and molecular diagnostics based on technology. Among these, the molecular diagnostics segment dominates the market. The molecular diagnostics segment is anticipated to demonstrate the high growth rate over the projection period.
The anticipated rise in the utilization of high-throughput PCR technology for detecting sexually transmitted diseases (STDs) is estimated to propel the NAAT segment. The cost-effectiveness, user-friendliness, and accuracy of this technology are projected to enhance its adoption. The majority of urine tests for STDs utilize NAATs due to their superior sensitivity. For instance,
Sexually spread dsiseases (STDs) are illnesses resulting from bacteria, viruses, or parasites that are spread via sexual intercourse, encompassing vaginal, anal, and oral sex. Vertical transmission can occur from mother to child during gestation, parturition, and lactation.
The illnesses induced by these viruses are asymptomatic; hence, prompt and precise diagnosis can mitigate further spread. To mitigate the impact of these diseases, regional and national government agencies are implementing measures to enhance knowledge about sexually transmitted disease testing, hence promoting the significance of sexual health.
A heightened awareness of these disorders among the general populace is resulting in a rise in the number of individuals seeking diagnosis. It is increasing the demand for tools and kits for testing sexually transmitted diseases, particularly in growing nations of Asia Pacific and Africa.
Along with market participants emphasizing the launch of innovative products such as point-of-care testing at relatively reduced prices is expected to propel market expansion over the projection period.
The sexually transmitted disease (STD) testing market experienced steady growth pre-2023, driven by rising awareness about sexual health, increased incidence of STDs, and expanding diagnostic technologies. The demand for STD testing services was propelled by government initiatives promoting early detection and prevention, as well as the increasing prevalence of infections such as HIV, chlamydia, gonorrhea, and syphilis.
Technological advancements including at-home testing kits and the rising adoption of point-of-care (POC) diagnostics further fueled market expansion. From 2019 to 2023, the market exhibited consistent growth in Asia Pacific and North America being key regions due to high STD rates and robust healthcare infrastructure.
Post-2024, the market is expected to grow significantly supported by ongoing innovations in diagnostic technology and increased focus on reducing the stigma surrounding STD testing. Enhanced telemedicine services and digital health platforms are likely to boost demand for at-home STD testing kits.
Implementing comprehensive sexual health programs in emerging markets and increased funding for disease prevention initiatives will contribute to market expansion. Artificial intelligence (AI) integration in diagnostics and improved healthcare access in developing regions are anticipated to drive growth.
Rising Prevalence of Sexually Transmitted Diseases (STDs)
One of the primary growth drivers for the STD testing market is the increasing incidence of sexually transmitted infections worldwide. Diseases such as chlamydia, gonorrhoea, syphilis, and human papillomavirus (HPV) have seen rising case numbers due to factors like unprotected sexual activities, multiple sexual partners, and lack of comprehensive sex education.
Public health organizations and governments are emphasizing early diagnosis and treatment to curb the spread of these infections, which has in turn driven demand for reliable testing services. The growing awareness of long-term complications associated with untreated STDs, such as infertility, cancer, and increased risk of HIV transmission, further incentivizes people to undergo routine testing.
Consequently, healthcare systems are adopting more robust testing mechanisms, making STD testing more accessible and accurate, particularly in regions with high infection rates.
Technological Advancements in Diagnostic Testing
Innovations in diagnostic technologies have greatly contributed to the expansion of the STD testing market. The development of highly sensitive and specific diagnostic methods, such as nucleic acid amplification tests (NAATs), has improved the accuracy and reliability of STD detection.
The availability of point-of-care (POC) tests and at-home testing kits has simplified the process, making it more convenient for individuals to get tested without visiting healthcare facilities. These advancements allow for faster detection and early intervention, reducing the time between diagnosis and treatment.
Digital health tools and telemedicine platforms have integrated STD testing into broader sexual health services, offering remote consultations and prescriptions. This has encouraged higher participation in routine testing, particularly among younger demographics who prefer discreet and convenient healthcare solutions.
The continued evolution of diagnostic technology is expected to enhance market growth, as new methods make testing more accessible, affordable, and efficient.
Government Initiatives and Public Health Campaigns
Government-led initiatives and public health campaigns have played a crucial role in promoting STD testing and awareness. Many countries have implemented national sexual health programs aimed at educating the public about STDs, reducing stigma, and encouraging regular testing. For instance,
A widespread public health campaigns through digital and traditional media channels focus on breaking down the stigma associated with STD testing motivating more individuals to seek out testing. As governments continue to recognize the importance of early detection and treatment to reduce healthcare costs and manage the spread of infections, they are likely to further invest in expanding STD testing infrastructure contributing to sustained market growth.
Social Stigma and Lack of Awareness
One significant restraint for the STD testing market growth is the persistent social stigma surrounding sexually transmitted diseases and their testing. Many individuals in conservative societies may avoid getting tested due to fear of judgment, shame, or embarrassment. This stigma can prevent early detection leading to delayed treatment and potentially increased transmission of infections.
The stigma can contribute to underreporting and inadequate public health responses even in more progressive regions. In addition, a lack of comprehensive sexual education in many parts of the world further exacerbates the issue, as individuals may not fully understand the risks of STDs or the importance of routine testing. This cultural and societal barrier reduces the overall uptake of testing services, slowing market growth despite advancements in testing technologies.
Expansion of At-Home STD Testing Kits
One of the most transformative opportunities in the STD testing market is the expansion of at-home testing kits. These kits offer a discreet, convenient, and accessible alternative to traditional clinic-based testing addressing the stigma and privacy concerns that often prevent individuals from seeking STD testing.
At-home kits typically allow users to collect samples in the privacy of their homes and send them to certified labs for analysis with results delivered electronically. This model not only reduces the psychological barrier to testing but also broadens access for individuals in remote areas or those with limited mobility.
The integration of telemedicine with these kits further enhances their appeal, enabling users to consult healthcare professionals and receive treatment remotely. As consumer demand for convenience and privacy grows, companies that develop accurate, reliable, and user-friendly at-home STD testing solutions stand to gain significant market share driving the next wave of growth in the sector.
Integration of Artificial Intelligence (AI) in Diagnostic
The integration of artificial intelligence (AI) in STD testing presents a significant opportunity for transforming diagnostics. AI-driven platforms can analyze large datasets and identify patterns in test results more efficiently than traditional methods, enabling fast and more accurate diagnosis. AI tools can also be used to improve test sensitivity and specificity, particularly for complex or hard-to-diagnose infections.
AI-based predictive models could assist in identifying high-risk populations and optimizing public health strategies for controlling STD outbreaks. These capabilities could dramatically reduce the time between infection, diagnosis, and treatment, ultimately lowering transmission rates.
The use of AI in diagnostics also opens doors for personalized healthcare, offering tailored testing solutions based on an individual's risk profile, medical history, or location. As AI technologies become more widespread in the healthcare industry, they are likely to revolutionize the STD testing market by improving diagnostic accuracy, accessibility, and overall patient outcomes.
The competitive landscape of the sexually transmitted disease (STD) testing market is characterized by the presence of several key players offering a range of diagnostic solutions.
Leading companies include Abbott Laboratories, Bio-Rad Laboratories, F. Hoffmann-La Roche, and Quest Diagnostics, which dominate the market through innovative technologies and extensive global distribution networks.
Leading companies invest in advanced diagnostic techniques like nucleic acid amplification tests (NAATs) and at-home testing kits catering to rising consumer demand for accurate and convenient testing. Small playersincluding Everlywell and LetsGetChecked, are gaining traction in the at-home testing space, driving competition in direct-to-consumer services. Strategic partnerships, product innovations, and expanding telemedicine platforms further intensify competition as companies strive to enhance accessibility and reduce testing costs.
Recent Industry Developments in the Sexually Transmitted Disease Testing
|
Attributes |
Details |
|
Forecast Period |
2024 to 2031 |
|
Historical Data Available for |
2019 to 2023 |
|
Market Analysis |
US$ Billion for Value |
|
Key Regions Covered |
|
|
Key Market Segments Covered |
|
|
Key Companies Profiled in the Report |
|
|
Report Coverage |
|
|
Customization & Pricing |
Available upon request |
By Product
By Application
By Technology
By Region
To know more about delivery timeline for this report Contact Sales

The market is estimated to be valued at US$17.08 Bn by 2031.
The market is projected to exhibit a CAGR of 7.9% over the forecast period.
BD, F. Hoffmann-La Roche Ltd, Hologic Inc., Abbott, are some of the key players operating in the market.
Technological advancements in diagnostic testing drives the market forward.
North America dominates the market for sexually transmitted disease testing.