ID: PMRREP10598| 194 Pages | 6 Oct 2025 | Format: PDF, Excel, PPT* | Healthcare

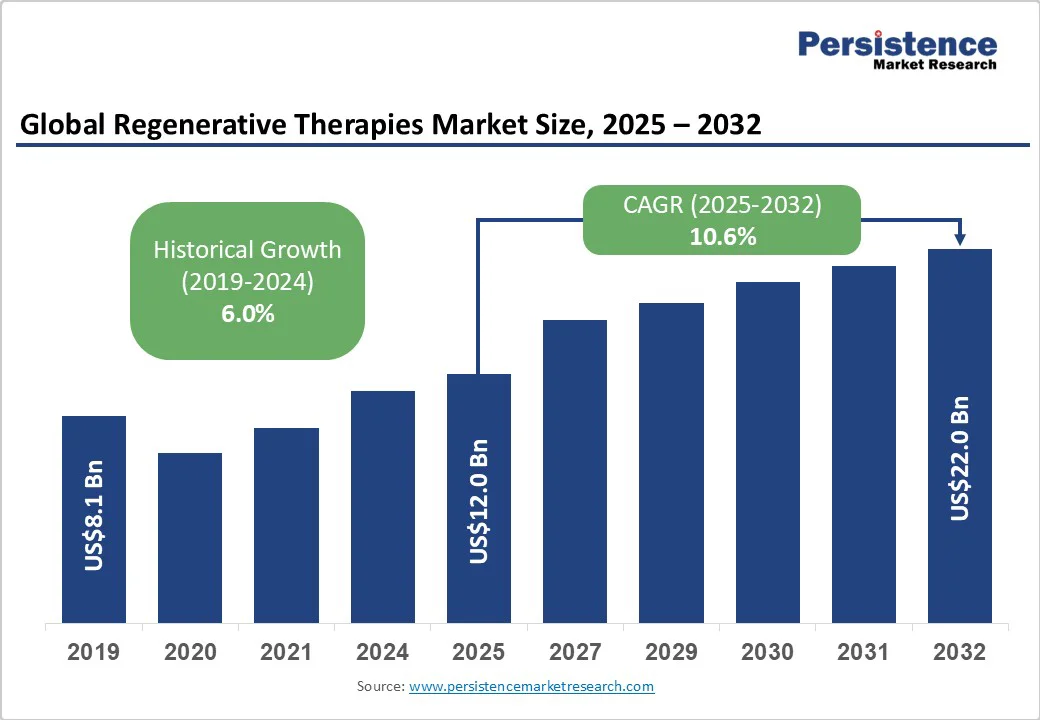
The global regenerative therapies market size is likely to be valued at US$12.0 Billion in 2025 and is estimated to reach US$22.0 Billion by 2032, growing at a CAGR of 10.6% during the forecast period 2025−2032, driven by the increasing prevalence of chronic diseases, continuous technological advancements, and expanding supportive regulatory frameworks. Innovations in stem cell therapies, gene editing, and tissue engineering underpin this expansion, supported by rising investments and expanding clinical applications.
| Key Insights | Details |
|---|---|
|
Regenerative Therapies Market Size (2025E) |
US$12.0 Bn |
|
Market Value Forecast (2032F) |
US$22.0 Bn |
|
Projected Growth (CAGR 2025 to 2032) |
10.6% |
|
Historical Market Growth (CAGR 2019 to 2024) |
6.0% |
The growing global burden of chronic diseases such as cardiovascular disorders, diabetes, neurodegenerative diseases, and cancer is the primary market driver. According to the World Health Organization (WHO), chronic diseases killed over 43 million people globally in 2021. These numbers bolster the demand for regenerative therapies that provide curative rather than symptomatic treatment. This trend accelerates clinical research and market adoption, with cancer-focused regenerative therapies constituting nearly 50% of current pipelines, fueling a rapidly expanding market segment.
Breakthroughs in stem cell research, CRISPR gene editing, tissue engineering, and biomaterial science have enabled the development of novel regenerative products with enhanced efficacy and safety profiles. The U.S. FDA’s approval of pluripotent stem cell-derived therapies, such as BlueRock Therapeutics’ DA01, exemplifies regulatory validation of these innovations. Coupled with advances in manufacturing automation, these developments lower production costs and improve scalability, driving accelerated commercialization globally.
Governments worldwide, especially in North America and Asia Pacific, have instituted expedited regulatory pathways and increased funding to support regenerative medicine R&D. For example, the U.S. has implemented the Regenerative Medicine Advanced Therapy (RMAT) designation to fast-track clinical trials. Public-private partnerships and venture capital investment surged to US$22.7 Billion in 2021, according to the Alliance for Regenerative Medicine, enabling accelerated development and broader market access.
The intricate manufacturing processes and the need for personalized treatments make regenerative therapies costly. High prices limit broad adoption, particularly in emerging markets. The evolving reimbursement landscape also presents uncertainties, contributing to a barrier that could slow market penetration by approximately 10-15% until cost-efficient solutions mature.
Despite positive regulatory movements, inconsistencies across regions, particularly in Asia Pacific and Europe, create approval delays and complicate global commercialization strategies. Additionally, ethical concerns related to stem cell sourcing and genetic modification pose ongoing risks, mandating rigorous compliance that can slow clinical trial progression by 6-12 months on average, impacting market timelines.
Asian economies, led by China, Japan, and India, represent lucrative growth frontiers driven by expanding healthcare infrastructure and growing patient populations. China regenerative medicine market is growing at over 20.0% CAGR, propelled by increasing government funding and rapid adoption of advanced therapies, potentially contributing an additional US$10 Billion to global revenues by 2030.
Integrating AI, machine learning, and 3D bioprinting with regenerative medicine unlocks new avenues for precise therapy development and customized treatments. The advent of AI-enabled drug discovery could reduce R&D timelines by up to 30%, enhancing pipeline efficiency and supporting faster time-to-market. Moreover, therapies targeting rare diseases and complex indications such as spinal cord injuries and autoimmune conditions remain underserved, presenting niches that could generate an incremental market size of US$5-7 Billion by 2032. Focused investment in these areas promises early mover advantages as competitors develop scalable solutions.
Cell therapy is set to continue to dominate the regenerative therapies market with an approximate 45.0% share in 2025, driven by its widespread clinical use, particularly in oncology and musculoskeletal disorder treatments. Stem cell therapies maintain leadership due to their proven clinical efficacy and extensive presence in the development pipeline, supported by a growing number of Phase II and III trials.
Gene therapy is emerging rapidly, forecasted to outpace other segments with a CAGR exceeding 30.0% through 2032. This acceleration is largely attributable to advancements in precision gene-editing technologies such as CRISPR-Cas systems and expanding regulatory frameworks that expedite approval processes. The transition from predominantly ex vivo approaches toward in vivo gene therapies is broadening therapeutic indications beyond cancers to include rare genetic diseases, cardiovascular disorders, and metabolic conditions. This expansion not only opens new treatment possibilities but also attracts significant investor and commercial interest, marking gene therapy as a pivotal driver of future market growth.
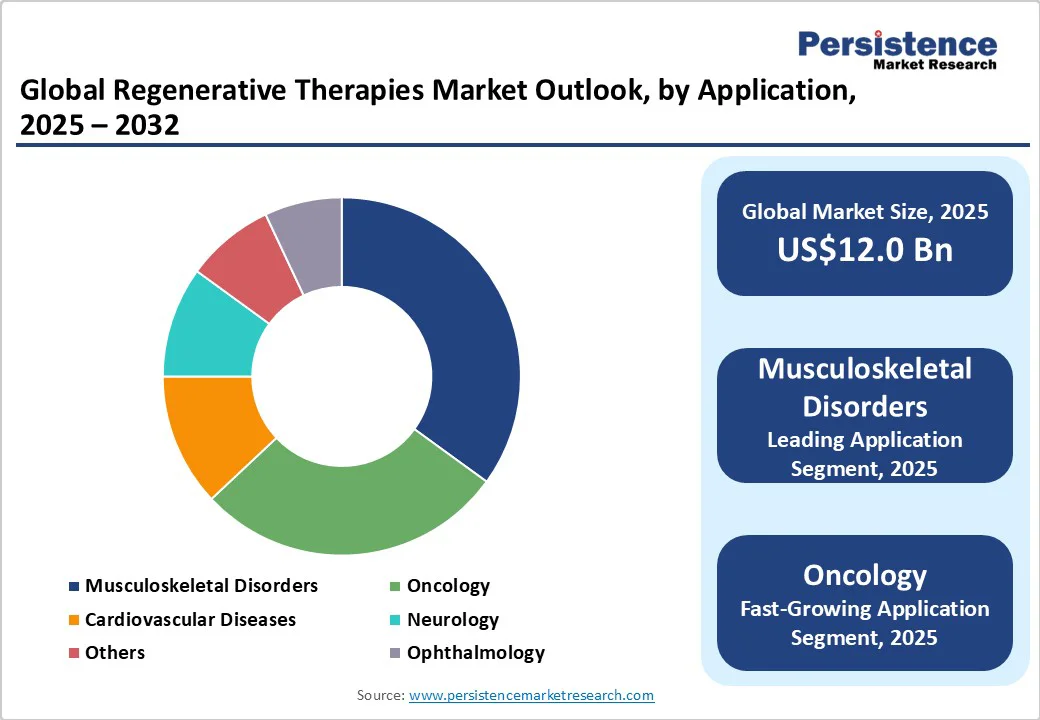
Musculoskeletal disorders are expected to hold the largest share, constituting nearly 35.0% of the regenerative therapies application market in 2025. This prominence stems from demographic shifts such as global population aging and increased prevalence of degenerative conditions such as osteoarthritis and spinal injuries. Osteoarthritis prevalence peaks in populations aged above 55 years, accounting for approximately 73.0% of cases worldwide, necessitating regenerative interventions focused on cartilage regeneration and bone repair. The urgent unmet needs among chronic musculoskeletal patients have catalyzed increased R&D investments, leading to innovative therapies targeting tissue regeneration and pain management.
Oncology is the fastest-growing application segment, with a projected CAGR exceeding 28.0%, propelled by breakthroughs in CAR-T cell therapies and other immuno-oncology modalities that are revolutionizing treatment paradigms. The rising global cancer incidence is intensifying the demand for personalized, curative interventions, with CAR-T therapies demonstrating substantial success in hematologic malignancies. This has triggered an influx of clinical trials and upper-level regulatory approvals in major markets, including the U.S. and Europe, driving significant commercial potential.
Hospitals currently dominate, accounting for about 55.0% of the revenue share in 2025. This leadership is underpinned by their comprehensive clinical infrastructure, high patient throughput, and capabilities to perform complex regenerative procedures such as CAR-T cell infusions and autologous stem cell transplantation. The multidisciplinary care teams and availability of specialized technological equipment allow hospitals to efficiently manage therapy administration and post-procedural monitoring.
Conversely, specialty clinics are emerging as the fastest-growing end-user segment, expanding at about 22.0% CAGR. Their growth reflects advantages such as lower operational overhead, improved patient convenience, and the increasing adoption of minimally invasive regenerative treatments and aesthetic procedures suitable for outpatient settings. These clinics capitalize on technological advancements, enabling simplified therapeutic delivery and facilitating access to regenerative treatments outside traditional hospital environments. This evolving landscape signals diversification of care delivery channels and expanding market access options.
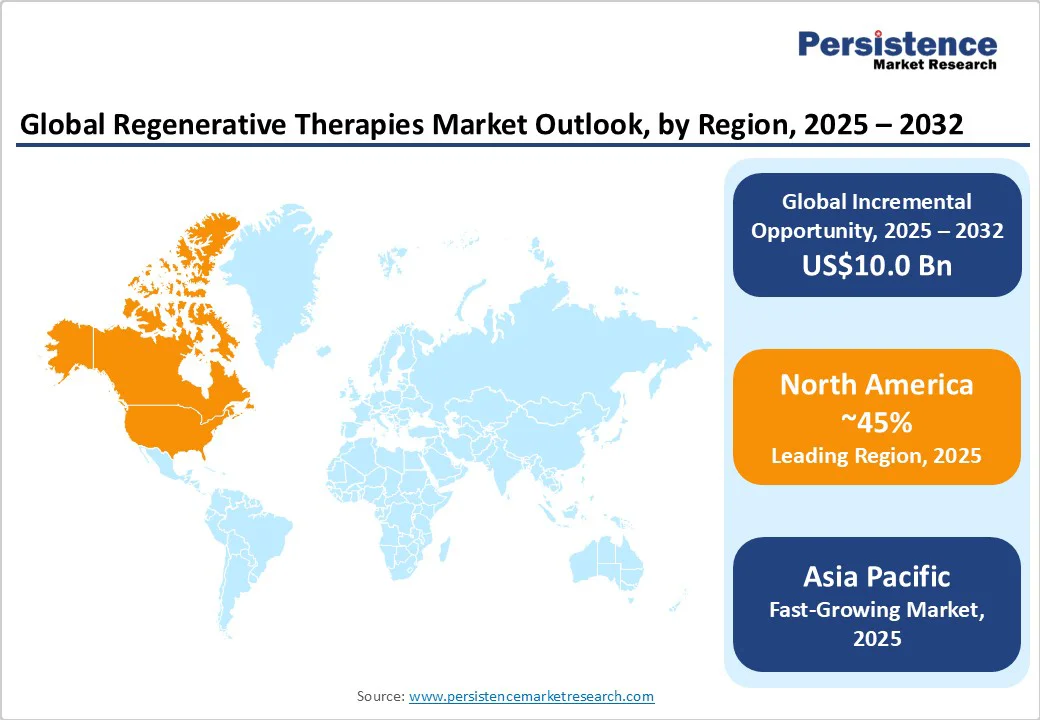
North America is the undisputed leader in the regenerative therapies market, commanding a 45.0% share valued at around US$19.1 Billion in 2025. The United States spearheads regional growth supported by forward-looking regulatory policies such as the FDA's Regenerative Medicine Advanced Therapy (RMAT) designation, designed to accelerate development and market entry of breakthrough therapies.
The U.S. invests over USD8 Billion annually in research and development, resulting in a dynamic innovation ecosystem characterized by frequent product launches, strategic partnerships, and a rich therapeutic pipeline comprising cell, gene, and tissue engineering modalities. Venture capital deployment remains high, nurturing startups with niche regenerative platforms and expediting commercialization efforts. Additionally, a rising incidence of chronic diseases, including cancer, diabetes, and neurodegenerative conditions, sustains demand, positioning North America as an innovation and market expansion hub.
Europe is anticipated to account for approximately 25.0% of the regenerative therapies market share, propelled by key contributors such as Germany, the U.K., France, and Spain. Regulatory harmonization under the European Medicines Agency (EMA) facilitates streamlined market access across member states, although approval timelines generally lag behind U.S. benchmarks, moderating growth rates to an estimated 18.0% CAGR through 2032.
Strong collaborations between academia and industry bolster translational research, particularly in orthopedics and cardiovascular applications, supported by increasing healthcare expenditure. Robust public-private partnerships and targeted funding initiatives also drive steady market expansion by supporting clinical trial activities and commercialization. Europe's focus on integrated healthcare delivery models and diverse patient populations ensures sustained demand for regenerative therapies in both urban and rural settings.
Asia Pacific emerges as the fastest-growing regional market with a leading CAGR through 2032. Growth is powered by expansive government support mechanisms, major investments in healthcare infrastructure, and cost efficiencies in biotechnology manufacturing and clinical trials relative to Western counterparts.
China leads the region, with the regenerative medicine market anticipated to exceed US$15 Billion by 2030, driven by proactive regulatory reforms and significant private sector involvement. Other countries in the region, including India and ASEAN members, are progressively aligning regulatory standards with global best practices, facilitating smoother market entry and international collaborations. The competitive landscape reflects a mix of established multinational corporations and local biotech firms, intensifying R&D activities and market penetration efforts. Furthermore, increasing middle-class population, rising prevalence of chronic diseases, and enhanced public healthcare awareness collectively contribute to sustained demand growth.
The global regenerative therapies market landscape is moderately consolidated, with the top 10 players commanding approximately 65% of revenue. Leading firms include Novartis, Pfizer, and BlueRock Therapeutics. Market concentration has intensified in cell and gene therapy segments, while tissue engineering remains more fragmented. Competitive positioning centers on innovation, leadership, and partnership-driven growth. Market leaders are prioritizing innovation through R&D, extensive collaborations, and geographic expansion. Cost leadership is less prevalent due to the high-value nature of therapies, while personalized medicine business models gain traction.
The regenerative therapies market is projected to reach US$12.0 Billion in 2025.
Key drivers include the rising prevalence of chronic diseases, ongoing technological advances, and evolving regulatory support.
The regenerative therapies market is poised to witness a CAGR of 10.6% from 2025 to 2032.
Innovations in stem cell therapies, gene editing, and tissue engineering, supported by rising investments and expanding clinical applications, are the key market opportunities.
Novartis, Pfizer, and BlueRock Therapeutics are some of the key market players.
| Report Attribute | Details |
|---|---|
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis |
Value: US$ Bn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
By Therapy Type
By Application
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author